Tusike
- 137
- 0
Homework Statement
Hi! I apologize in advance for my knowledge of vocabulary in this topic:)
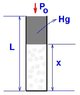
OK, so we have a Melde-tube 55 cm long, T=293K, with the top of it open, and a 20cm Hg part cutting off 30cm of air when is equilibrium. The original problem asked how much we can heat the air so that no Hg would come out of the tube, that's fairly easy.
My question is, what happens if we heat the air beyond that temperature? Because sure, we increased the temperature, but the Hg came out, and so the pressure decreased! I'm interested in some sort of V-T diagram beyond this point, or how to calculate the work done by the air after all the Hg came out of the tube.
Homework Equations
pV=nRT=NkT
dE = Q + W
The Attempt at a Solution
I really don't have much idea but I'll post the solution to the original problem so it'd be easier to calculate from beyond that point:
Gay Lussac 1: V1 / T1 = V2 / T2 -> L1 / T1 = L2 / T2
where L1 = 30cm, T1 = 293K, L2 = Lmax = 35 cm, T2 = ? = 35 / 30 * 293 = 342K.
Thanks for any help!