IcedCoffee
- 20
- 4
I'm confused about the mathematics that led to the equation 4.5.9.
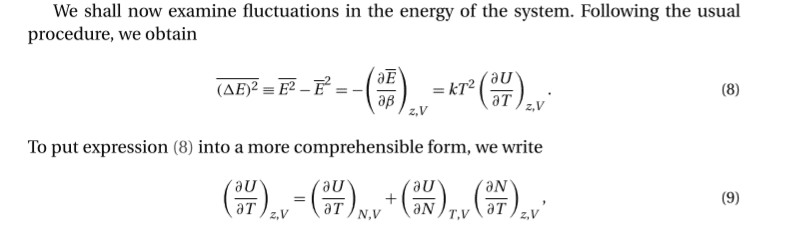
Specifically, I'm confused about what the variables that describe U are.
From the equation
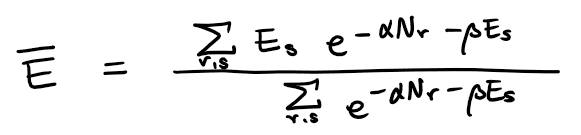
I think temperature T(through beta), chemical potential (through alpha), V (through E_s) and N (through... restriction on the summation?) defines the (average) internal energy U,
but how should I do the differentiation? What I ended up was that the chemical potential dependence can be changed to the dependence on the fugacity z so that
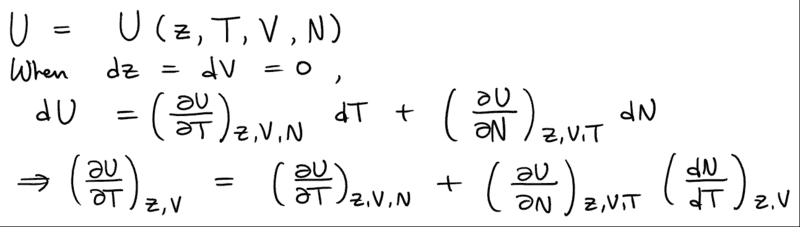
but it doesn't seem to be exactly eq.4.5.9 to me.
Specifically, I'm confused about what the variables that describe U are.
From the equation
I think temperature T(through beta), chemical potential (through alpha), V (through E_s) and N (through... restriction on the summation?) defines the (average) internal energy U,
but how should I do the differentiation? What I ended up was that the chemical potential dependence can be changed to the dependence on the fugacity z so that
but it doesn't seem to be exactly eq.4.5.9 to me.