Graham87
- 72
- 16
- Homework Statement
- See pic
- Relevant Equations
- See pic
How do you know which binding energy shell to use? In the solution it uses K and L2. Why specifically L2 and not L3 or L1 for example?
And what should I do with the information to omit electrons lower than 20kev? I initially thought that meant to omit the electron binding energies lower than 20kev. But L2 which is lower than 20kev is included, so which expression represents electron energy? If it is ΔE - B(L) then shouldn’t L3 be included since it also has a higher energy than 20kev?
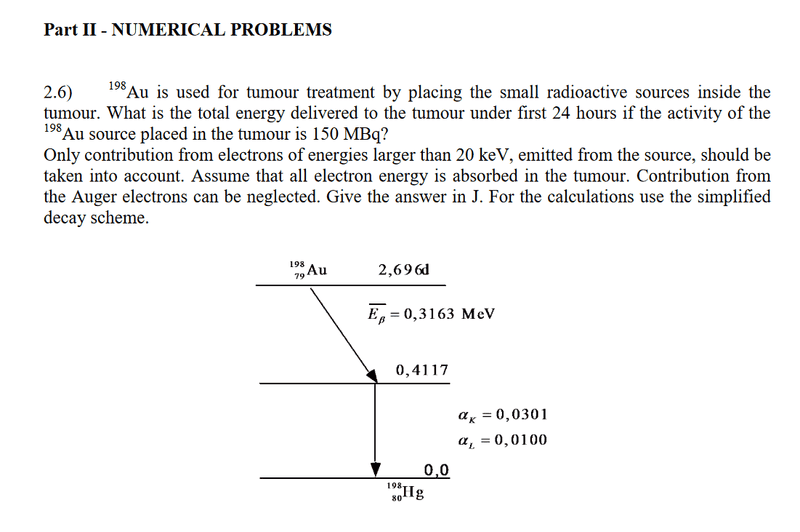

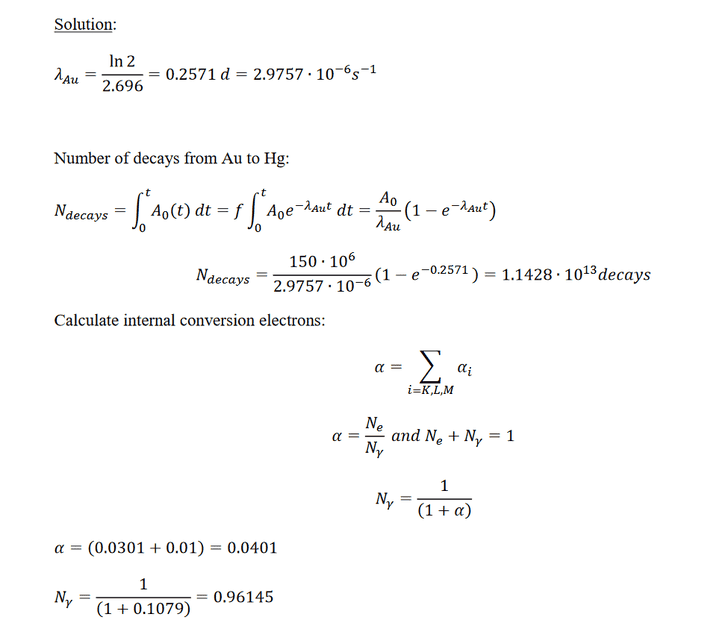
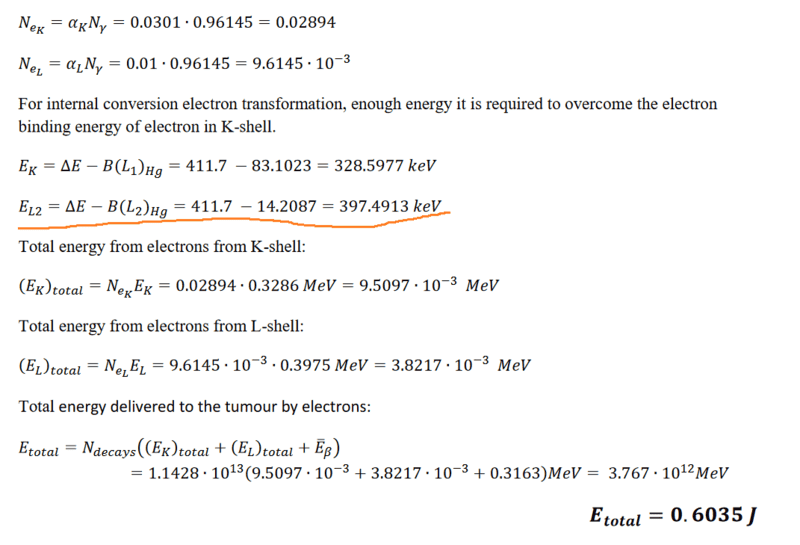
And what should I do with the information to omit electrons lower than 20kev? I initially thought that meant to omit the electron binding energies lower than 20kev. But L2 which is lower than 20kev is included, so which expression represents electron energy? If it is ΔE - B(L) then shouldn’t L3 be included since it also has a higher energy than 20kev?