leopard
- 123
- 0
Is this the E or Z isomer of 3-isopropyl-2-heptene?
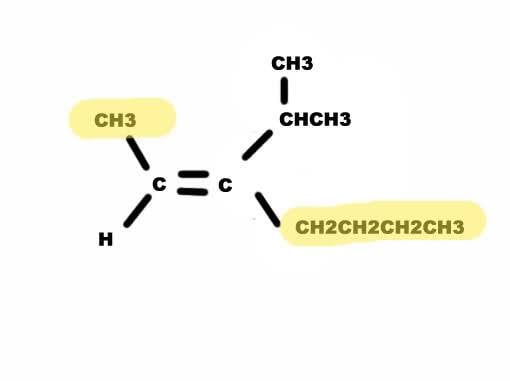
My book says Z, but I would think it's E. On the left, there is no doubt which group has higher priority. I would believe that on the right side, the lower group has the higher priority because there is a longer chain of C-atoms.
My book says Z, but I would think it's E. On the left, there is no doubt which group has higher priority. I would believe that on the right side, the lower group has the higher priority because there is a longer chain of C-atoms.