unscientific
- 1,728
- 13
I don't really understand the explanation given in Binney's text about:
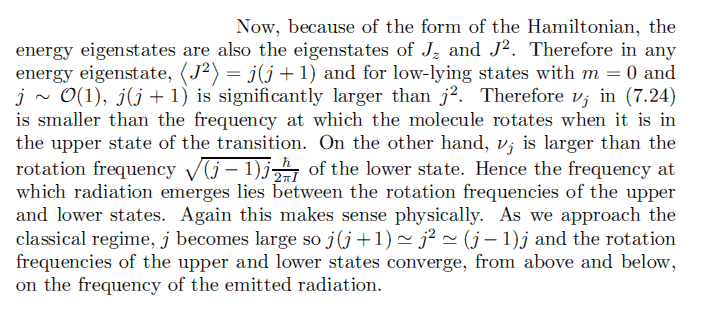
Hamiltonian is given by:
H = \frac{\hbar^2}{2} \left( \frac{J_x^2}{I_x} + \frac{J_y^2}{I_y} + \frac{J_z^2}{I_z} \right)
Orient axes such that ##I_x = I_y = I##.
H = \frac{\hbar^2}{2} \left( \frac{J^2}{I} + J_z^2(\frac{1}{I_z} - \frac{1}{I})\right)
Energy is given by:
E_{jm} = \frac{\hbar^2}{2} \left[ \frac{j(j+1)}{I} + m^2(\frac{1}{I_z} - \frac{1}{I}) \right]
We are only interested in states:
E_{jm} = \frac{\hbar^2}{2I} j(j+1)
Emitted energy and frequency are:
\Delta E_p =\pm (E_j - E_{j-1}) = \pm j\frac{\hbar^2}{I}
v_j = j\frac{\hbar}{2\pi I}
Let's try to analyze the explanation here.
1. Yes, energy, Jz and J2 share the same eigenstates ##|j, m>##.
2. <J2> = j(j+1) : Yes, since that is the eigenvalue and eigenvalue correspond to real observables.
3. Why do low lying states with ##m = 0## and ##j~O(1)## lead to: ## j(j+1) >> j ##? Firstly, doesn't low lying states correspond to a low ##j##? And what does m have to do with anything? ##m## was defined as the eigenvalue of Ji and ##j = m_{max}##
The rest of the argument doesn't make any sense at all..
Hamiltonian is given by:
H = \frac{\hbar^2}{2} \left( \frac{J_x^2}{I_x} + \frac{J_y^2}{I_y} + \frac{J_z^2}{I_z} \right)
Orient axes such that ##I_x = I_y = I##.
H = \frac{\hbar^2}{2} \left( \frac{J^2}{I} + J_z^2(\frac{1}{I_z} - \frac{1}{I})\right)
Energy is given by:
E_{jm} = \frac{\hbar^2}{2} \left[ \frac{j(j+1)}{I} + m^2(\frac{1}{I_z} - \frac{1}{I}) \right]
We are only interested in states:
E_{jm} = \frac{\hbar^2}{2I} j(j+1)
Emitted energy and frequency are:
\Delta E_p =\pm (E_j - E_{j-1}) = \pm j\frac{\hbar^2}{I}
v_j = j\frac{\hbar}{2\pi I}
Let's try to analyze the explanation here.
1. Yes, energy, Jz and J2 share the same eigenstates ##|j, m>##.
2. <J2> = j(j+1) : Yes, since that is the eigenvalue and eigenvalue correspond to real observables.
3. Why do low lying states with ##m = 0## and ##j~O(1)## lead to: ## j(j+1) >> j ##? Firstly, doesn't low lying states correspond to a low ##j##? And what does m have to do with anything? ##m## was defined as the eigenvalue of Ji and ##j = m_{max}##
The rest of the argument doesn't make any sense at all..
Last edited: