TheFistGuy
- 7
- 1
Hello guys!
I'am having trouble getting my head around the physics of evaporation in quite specific situation.
So i have a closed container with opening in the bottom (cross-section in picture below). Let's say that water has a constant temperature of 80°C and container has a temperature 90°C, therefore the water cannot condense on the inner edges of the container. Outside the container is air with sea-level pressure.
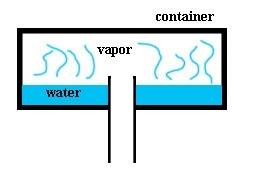
So water evaporates and since vapor is lighter than air and it rises up. As it builds up in the container it should create some pressure (right?). Then due to the pressure the vapor should start escaping through the bottom (right?). So the first question is how much pressure is needed for the vapor to escape through the bottom?
And since vapor pressure builds up, that should slow the rate of evaporation. Is there any way to calculate this?
I will appreciate any help or links to related problems. Thanks!
I'am having trouble getting my head around the physics of evaporation in quite specific situation.
So i have a closed container with opening in the bottom (cross-section in picture below). Let's say that water has a constant temperature of 80°C and container has a temperature 90°C, therefore the water cannot condense on the inner edges of the container. Outside the container is air with sea-level pressure.
So water evaporates and since vapor is lighter than air and it rises up. As it builds up in the container it should create some pressure (right?). Then due to the pressure the vapor should start escaping through the bottom (right?). So the first question is how much pressure is needed for the vapor to escape through the bottom?
And since vapor pressure builds up, that should slow the rate of evaporation. Is there any way to calculate this?
I will appreciate any help or links to related problems. Thanks!