hassony
- 1
- 0
Threads moved from a technical forum so no Homework Template is shown.
TL;DR Summary: Quantum number of a state
What is the largest quantum number of a state of the Li3+ ion with an orbital radius equal to 60 A?
I tried solving the question as below
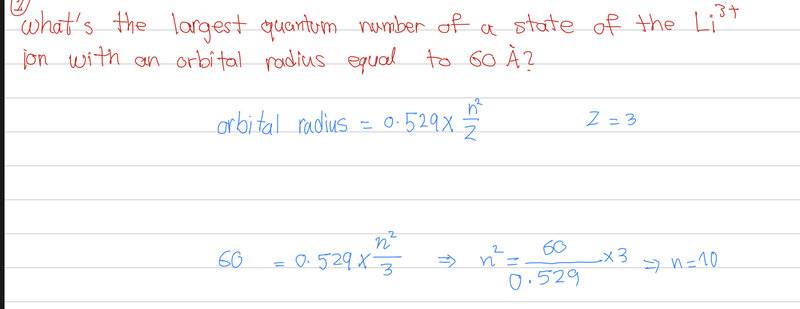
What is the largest quantum number of a state of the Li3+ ion with an orbital radius equal to 60 A?
I tried solving the question as below