- 2,376
- 348
Introduction
The student of thermodynamics, as they consider pistons and ideal gasses and such, often begin to grasp the nature of entropy only to find as they delve deeper that grasp slips away. In the deeper analysis of thermodynamic systems via statistical mechanics this grasp may slip away entirely.
“How can my cup of hot tea, have a given entropy from instant to the instant when at any given instant it is in some exact physical state whether I know which one it is or not?” “If entropy is a measure of disorder then what makes one state more ordered than another? By what divine aesthetic is order measured?”
One begins to think entropy is merely an on-paper quantity describing only an aspect the probability distributions of various states of reality. But, when a sip of that hot tea burns the tongue the immediately physical nature of entropy becomes very hard to deny.
Thermodynamics and Entropy
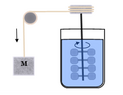
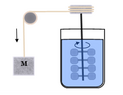
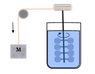
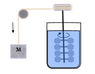
Joule’s...
Continue reading...
Attachments
Last edited by a moderator: