srinaath
- 51
- 2
I am trying to understand the working of simple hydrogen fuel cell.
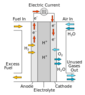
We have anode (negative terminal), cathode (positive terminal), catalyst at anode to separate negative and positive ions of hydrogen atoms and electrolyte which allows only positive ions.
We will be sending hydrogen fuel at anode where both positive and negative ions separated. electrons goes through external wire providing electricity. Also i read that we will be sending air to cathode. Why is that required? I mean without air won't the system still work? positive ions will still travel though electrolyte
Kindly provide me some insight.
We have anode (negative terminal), cathode (positive terminal), catalyst at anode to separate negative and positive ions of hydrogen atoms and electrolyte which allows only positive ions.
We will be sending hydrogen fuel at anode where both positive and negative ions separated. electrons goes through external wire providing electricity. Also i read that we will be sending air to cathode. Why is that required? I mean without air won't the system still work? positive ions will still travel though electrolyte
Kindly provide me some insight.