You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Melting point Definition and 78 Threads
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.
When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point."
View More On Wikipedia.org
When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point."
View More On Wikipedia.org
-
W
I Melting point of graphite and diamond?
I was conversing with ChatGPT when I asked which material has the highest melting point, and it answered "tungsten" (giving its melting temperature). It so happened that in the previous question the melting point of graphite had come up, and it was listed as higher than that. When I asked which...- Warp
- Thread
-
- Tags
- Diamond Graphite Melting point
- Replies: 3
- Forum: Classical Physics
-
R
Calculate the Temperature and Pressure of a melting point
Adiabatic increase in pressure implies Tds=0, can someone tell me how to proceed?- romanski007
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-
H
Thermodynamics: Why does salt lower the melting point of ice?
There was a question on "Why salt lower the freezing point of water?"I found the following answer."Thermodynamics teaches that a loss of entropy can be overcome by a gain in so called enthalpy". The loss of entropy by freezing the solution canbe over come at temperature much below 0 degree C...- hariharan venkatasu
- Thread
- Replies: 21
- Forum: Chemistry
-
K
"Heat resistance temperature" and melting point?
I've got a kitchen bowl made of polypropylene, it states that the "heat resistance temperature is 90 degree cel", while on Google, I found that the melting point of PP is 160 degree cel. I understand that melting point is the temperature in which the atoms/molecules change its state from solid... -
Z
Conductor that has the highest melting point
Hoping for feedback regarding engineered conductors that has high melting points (>4000C). Does anyone perhaps know of such metallic alloys or conductive material? (please, no 2D/1D materials -- exotic materials/alloys are OK, just has to be able to be made in bulk!) Thank you!- ZeroFunGame
- Thread
- Replies: 10
- Forum: Materials and Chemical Engineering
-
K
Heating up plastic before its melting point -- could it be bad?
I am interested in a water heater found in consumer market, however, it's made of kind of plastic, I often "feel" that there must be some problem heating up something like plastic. I understand that it should be tested and safe before putting into the market, however, I still wish to know -...- kenny1999
- Thread
- Replies: 3
- Forum: Materials and Chemical Engineering
-
T
Crystal with two different melting points
It turns out that the solid form is the same for the two situations but the liquid is two different isomers depending on external conditions during melting. https://physicstoday.scitation.org/do/10.1063/PT.6.1.20190606a/full/ -

B Quartz vs. Glass -- why do they have different melting points?
Why quartz has a higher melting Temperature than glass? Which one is harder and why?- Edge5
- Thread
- Replies: 4
- Forum: Other Physics Topics
-
H
B The physical properties of diamonds: no melting point?
I had heard an opinion from my high school teacher, but I can't understand?? "An experimental record of a French scientist. He heats the diamond and sublimates it. After cooling, it turns back to solid barbecue carbon. In this case, although there is a physical transition, the substance seems...- h_hin
- Thread
- Replies: 4
- Forum: Other Physics Topics
-
D
Why does the melting point of aspirin increase
I used sailcylic acid and acetic anhydride to produce aspirin and preformed recrystalisation to purify the aspirin. I need to evaluate why I have a higher/lower melting point. In this case, the melting point was 143°C whereas the true meling point is 135°C. I thought one of the reasons my...- Daniel2244
- Thread
- Replies: 19
- Forum: Chemistry
-

Heat transfer, how long it takes ice to reach melting point
Homework Statement A 0.25 kg piece of ice at -30 C is warmed by an electric heater and the following graph of temperature is produced. Assume that there has been no loss of energy to the surroundings. - Use the info on the graph to determine the power output of the heater - Explain how long...- Humbleness
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
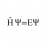
In which temperature range does WF_6 melt?
Hi Everyone I'm studying material Engineering and I'm currently preparing chemistry for the summer exams. Now, there is an old exam question which I don't know how to solve: "In which temperature range does ##[W^{+VI}F_{6}^{-I}]## melt?" My solution: Well, the 18-Electron rule is not...- H Psi equal E Psi
- Thread
- Replies: 5
- Forum: Engineering and Comp Sci Homework Help
-
E
Relation between Young's modulus and the coefficient of thermal expansion
Is it true for all material that if young's modulus is high then melting point will be high and coeff of thermal expansion will be lower? Any example that doesn't follow the above statement. Many Thanks- engnr_arsalan
- Thread
- Replies: 2
- Forum: Materials and Chemical Engineering
-
J
How Much Power Is Needed to Melt Lead?
This question is purely theoretical, so don't worry about safety or doability. Is there a way of calculating how many watts it takes to heat different metals? To be specific, how many amps and bolts it would take to get lead to its melting point. Thanks in advance.- JoeSalerno
- Thread
- Replies: 14
- Forum: Electrical Engineering
-
D
Melting point and boiling point
Why there is huge difference in M.P. and B.P. Of Oxygen (M.P. - 54K,B.P.- 90K) and sulphur (M.P. - 393 K, B.P. - 718 K)- Dragosagaragarwal
- Thread
- Replies: 1
- Forum: Chemistry
-
M
Correlating density and melting point in alkenes
Quoted data for cis and trans 1,2 dichloroethenes shows cis has higher density but lower melting point than trans. How can this be explained? Packing arguments are clearly unable to rationalise these observations. Other 1,2 disubstituted ethenes show similar pattern. Thank you.- Miffymycat
- Thread
-
- Tags
- Density Melting Melting point Point
- Replies: 4
- Forum: Chemistry
-
T
Why does arsenic sublimate, while gallium melts?
What does it tell you about a substance? A hight melting and boiling point means that the molecules hold together strongly, as in tungsten. A low melting point, as in hydrogen, means they're kinda loose. But what does the distance between them tell you? Something something entropy yadda yadda... -
A
What is the latent heat for medium carbon steel
I'have searched a lot but could not find latent heat for any kind of medium carbon steel for latent heat from solid to liquid and from liquid to gas. Also I want to know the corresponding melting point and boiling point for that know of steel. Any link for a table would be greatly appreciated...- Addison1
- Thread
- Replies: 1
- Forum: Materials and Chemical Engineering
-
Mixed Melting Point of Two Pure Solid Crystals
I understand why an impure solid will have a decreased melting point, however, in my recent organic chemistry lab section I mixed two finely ground pure solids together and observed that the mixture melted at a lower temperature than either pure solid. I understand this in principle, but...- Mike Dacre
- Thread
- Replies: 7
- Forum: Chemistry
-

Can I Easily Buy a Titanium Plate Instead of Melting It?
Titanium's melting point is between 1,600 and 1,700 degrees Celsius. What sort of equipment would I require to melt it? Is there something I can buy and install in my own little area that could melt titanium? Would I be able to power it or can this only be pulled off in a factory? Thanks.- Metals
- Thread
-
- Tags
- melting point titanium
- Replies: 7
- Forum: Materials and Chemical Engineering
-
B
Connection between increase in pressure and melting point
Hey guys What's the connection between an increase in pressure and the melting point of ice? Ice contracts as it melts so does an increase in pressure reduce or increase the melting point?- Bassee
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
A
Why do sugars have higher boiling points than water?
I recently heard about an interesting chemistry experiment I have yet to try. It involves boiling soda and watching the water rise and the sugars remain at the bottom of the pot. The reason being is sugars have higher boiling points than water. Thus, the water boils first, heats up, and rises to... -
J
Aluminum structure modifications for use near a very hot fire
I'm building an frame out of solid 5/8 inch thick aluminum square bars. this frame will be around a fire temps close to melting points but there will be insulation in make sure it won't melt. I was thinking about drilling holes in the free space of the aluminum to reduce it's weight and if you...- Jason White
- Thread
- Replies: 15
- Forum: Mechanical Engineering
-

Lead's melting point as a function of pressure (Thermo')
Homework Statement When lead is melted at atmospheric pressure, the melting point is 600K, the density decreases from 1.101x104 to 1.065x104 kgm-3. The latent heat is 24.5 kJ kg-1 Estimate the melting point of lead at a pressure of 100 atm. Homework Equations I haven't been able to find any...- Robsta
- Thread
- Replies: 15
- Forum: Introductory Physics Homework Help
-

What material has the lowest melting point?
What material has the lowest melting point? I'm doing an interesting research project of finding an invisible ink. My purpose is to find a material on which I can write on and has a very low melting point. I want to write on this kind of material by using my invisible ink. From my research...- INFINITE952
- Thread
- Replies: 4
- Forum: Materials and Chemical Engineering
-

Why does copper have a higher melting point than aluminium?
Why does copper have a higher melting point than aluminium? Why does the melting point increase as you go down groups anyway? Aluminium has higher charge (+3) and a smaller radius, meaning that there is a higher charge density and thus stronger forces of attraction to the lattice. While copper...- KingCrimson
- Thread
- Replies: 2
- Forum: Chemistry
-
T
List compounds in order of increasing melting point
Homework Statement The Attempt at a Solution My answer was C but the correct answer is A. My reasoning is that they are all bonded to chlorine, so the further away the element is from chlorine on the periodic table, the more ionic the compound is and the higher the melting point is. The...- toothpaste666
- Thread
- Replies: 15
- Forum: Biology and Chemistry Homework Help
-

How can neon have a melting point?
I just read that sodium has a higher melting point than neon. As neon is a noble gas, how can it have a melting point if it isn't a solid? -
H
Explaining Pressure: Boiling & Melting Point Changes
So at increased pressure (increased atmospheric), the boiling point of water Increases because the atmospheric pressure goes up. But at increased pressures, the melting point of a substance decreases! The explanation i'v seen is that it is due to le chatlier's principle. Ice is less dense...- Hereformore
- Thread
- Replies: 2
- Forum: Mechanics
-
F
Melting point of ice decreases with increase in pressure
Can someone please explain why the melting point of ice decreases with increase in pressure? I understand that generally speaking for a solid, greater the pressure applied, greater energy needed to break the bonds to change state and hence greater the temperature (melting point). What is the...- Fiona Rozario
- Thread
- Replies: 1
- Forum: Mechanics
-
D
Standard Entropy of a liquid at melting point with no S(298.15) given
Homework Statement Vapour pressures of a liquid have been measured and fit to the following equation: Log10 P (mmHg) = -3571/T + 8.999 The melting point has been determined to be 392.7 K. A Cp value given for the liquid is 250 J/mol K and the ΔSvap is 117.20 J/mol K Homework Equations...- DiffusConfuse
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
D
Standard Entropy for Liquid at Melting Point
Homework Statement The vapour pressures of a liquid have been measured and fit to the following equation: Log10 (mmHg) = -3571/T + 6.124 The melting point has been determined to be 392.7 K. Calculate the standard entropy of the liquid at the melting point. Homework Equations...- DiffusConfuse
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
D
Very quick question regarding melting point and purity
Homework Statement For the following melting point, indicate what might be concluded regarding the sample purity: Melting point: 147ºC (dec.) Homework Equations I assume that (dec.) means that the sample decomposed. The Attempt at a Solution I am wondering if this means that...- daselocution
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
A
Why does the melting point of graphite is higher than diamond?
In diamond valence electrons are fully covalently bonded.But in graphite only three are covalently bonded while one electron is freely moving.So it seem that melting point of diamond should be higher than that of graphite because in diamond we should break four covalent bonds while in graphite... -
D
Predicting the Melting Point of Stereoisomers: A Scientific Inquiry
The melting point of N-acetyl-DL-alanine is 137◦Cand that of N-acetyl-D-alanine is 125◦C. What would you expect the melting point of N-acetyl-L-alanine to be or is this impossible to predict? Why? I thought that to approach this problem all that needed to be done is take the average of...- disneychannel
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 8
- Forum: Chemistry
-

The Melting Point of Diamonds: Burning or No Burning?
I heard something that I adopted as a rule some years ago: "Every combustion produces H2O and CO2". I used it blindy until hydrogen burning came to mind. Clearly, H2 does not produce CO2. Now, what happens when a diamond reaches its melting point, and, does it burn afterwards? If so, what kind...- Weissritter
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 8
- Forum: Chemistry
-
W
Temp & Melting Point of Water: Pressure Effects Explained
assuming water at atmospheric conditions, now if the pressure is reduced, at some lower point of pressure, water starts to boil.at this point, will the temp of water change and why?- wave525
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 12
- Forum: Other Physics Topics
-
P
What Is the Difference Between Triple Point and Melting Point?
Hello, Im new here and I hope someone of you can answer this probably trivial question. I tried to find the answer in many of phyisc/termodynamic texbooks but in vain. http://imageshack.us/photo/my-images/62/123002h.jpg/ http://imageshack.us/photo/my-images/62/123002h.jpg/ According to...- Paul Snasel
- Thread
- Replies: 2
- Forum: Thermodynamics
-
E
Effect of pressure on Melting point
Hey, I have read that INCREASING the external pressure on solids INCREASES their melting point.(except for ice) WHY DOES THIS HAPPEN? (This is similar to the effect on Boiling point which rises when external pressure is increased. I know this happens because boiling takes place at temp...- emailanmol
- Thread
- Replies: 9
- Forum: Mechanics
-
S
Thermal stability vs melting point
An ionic compound is more thermally stable when the metal cation is more reactive. So its harder to decompose. But then what's the difference between melting and decomposition? Also, when an compound is harder to decompose, does it mean its melting point is higher? Because even though Na2O... -
S
How to tell if ionic compound has a higher melting point?
I know that MgO has a higher melting point than NaCl as Mg is Mg2+ while O is O2- while Na is Na+ and Cl ia Cl-. but in CuCO3 and Na2CO3, Na2CO3 has a higher melting point compared to CuCO3. So I'm not sure how to tell which ionic compound will have a higher melting point than another when they... -
S
Boiling and melting point of impure substances
They say that an impure substance has an increased boiling point and reduced melting point (i know that they will melt/boil at a range of temperature). But does impurity mean that its melting and boiling point is higher than the substance itself or it doesn't matter? I think that the impurity... -
P
Is there a type of plastic that has a low melting point or low soft temperature?
Is there a type of plastic that has a low melting point or better a low soft temperature? What we need is to blow up a ball (3-7 cm in diameter), cover the ball except a 6mm hole, let it dry, and then removes the plastic ball through the 6mm hole. So to remove the plastic it needs to be soft...- peterje
- Thread
- Replies: 9
- Forum: Materials and Chemical Engineering
-
I
Why is there a difference ?Melting Point of Lead & Tungsten: Find RMSA
Homework Statement Hi all, I have a question which is regarding melting point. I know that lindemann's criterion doesn't actually describe the phenomena of melting but is an approximation of what maybe occurring/ an intuitive approach. So the question is in a tabulated form for lead and...- ibysaiyan
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 1
- Forum: Advanced Physics Homework Help
-
P
Correlation between metal strength and melting point?
I am unsure of this matter and so I am curious, do stronger metals more than likely have higher melting/heating points? Does a metal that takes more force/pressure to break or snap have a higher melting/heating point as well? Do metals/alloys like steel or titanium have higher melting points...- Physics quest
- Thread
- Replies: 5
- Forum: Other Physics Topics
-
G
Melting point of the naphthalene [something went wrong]
hello, Last day in school we were heating solid naphthalene until it melted, but the temperature didn't stop at the melting point. if anybody has any idea of what could happen, please tell me, maybe we didn't do it correctly, or the test tube was dirty or whatever... Thanks- germangb
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 2
- Forum: Chemistry
-
Y
Impurities on melting point and boiling point of water
When impurities was added to the water, it tends to increase the boiling point of the water to 102 degree celcius and lower the melting point of the water to -2 degree celcius! Why this happen? Is it because the impurities tends to absorb the heat supplied to boil the water causing it to take in... -
A
Higher melting point, PH3 or NH3?
Hi everyone, I have a question for all of you In an exercise of chemistry it's written to find the compound, between every couple, which has the highest melting point. The three couples are: H20 H2S; KBr CF4, NH3 PH3. In the first case water has higher melting point because the bond is...- ANDR3W
- Thread
-
- Tags
- Melting Melting point Point
- Replies: 4
- Forum: Chemistry
-
C
Melting point of lead at 100atm
Homework Statement Homework Equations use clausius-clapeyron equation: \frac{dp}{dt} = \frac{L}{T(V_{2}-V_{1})} which can be rearranged (i think) in the following: p = p_{0}+\frac{L}{\Delta V}ln(\frac{T}{T_{0}}) The Attempt at a Solution so using the above equation i solved...- curto
- Thread
-
- Tags
- Lead Melting Melting point Point
- Replies: 14
- Forum: Introductory Physics Homework Help
-
E
Effect of pressure on melting point.
Is there a quantifiable value for a given substance that correlates to the degree by which pressure has an effect on the element or substance melting/boiling point? Allow me to elaborate. If the substance in question is known (silicon dioxide, for example), how could we calculate the...