You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Schrodinger equation: Definition and 564 Discussions
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of the subject. The equation is named after Erwin Schrödinger, who postulated the equation in 1925, and published it in 1926, forming the basis for the work that resulted in his Nobel Prize in Physics in 1933.Conceptually, the Schrödinger equation is the quantum counterpart of Newton's second law in classical mechanics. Given a set of known initial conditions, Newton's second law makes a mathematical prediction as to what path a given physical system will take over time. The Schrödinger equation gives the evolution over time of a wave function, the quantum-mechanical characterization of an isolated physical system. The equation can be derived from the fact that the time-evolution operator must be unitary, and must therefore be generated by the exponential of a self-adjoint operator, which is the quantum Hamiltonian.
The Schrödinger equation is not the only way to study quantum mechanical systems and make predictions. The other formulations of quantum mechanics include matrix mechanics, introduced by Werner Heisenberg, and the path integral formulation, developed chiefly by Richard Feynman. Paul Dirac incorporated matrix mechanics and the Schrödinger equation into a single formulation. When these approaches are compared, the use of the Schrödinger equation is sometimes called "wave mechanics".
View More On Wikipedia.org
The Schrödinger equation is not the only way to study quantum mechanical systems and make predictions. The other formulations of quantum mechanics include matrix mechanics, introduced by Werner Heisenberg, and the path integral formulation, developed chiefly by Richard Feynman. Paul Dirac incorporated matrix mechanics and the Schrödinger equation into a single formulation. When these approaches are compared, the use of the Schrödinger equation is sometimes called "wave mechanics".
View More On Wikipedia.org
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 31: Solution of the stationary-state Schrodinger equation for a SHO
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
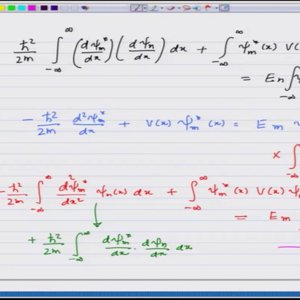
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 32: Equivalance of Heisenberg and the Schrodinger formulations I
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
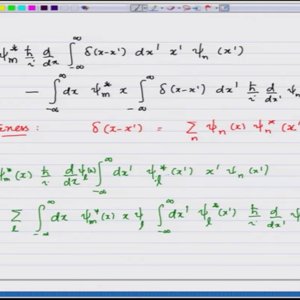
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 33: Equivalance of Heisenberg and Schrodinger formulations II
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 34: Born interpretation of the wavefunction
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 35: Uncertainty principle and its simple applications
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 36: Time dependent Schrodinger equation
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
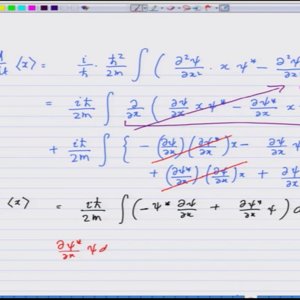
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 37: Ehrenfest theorem
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
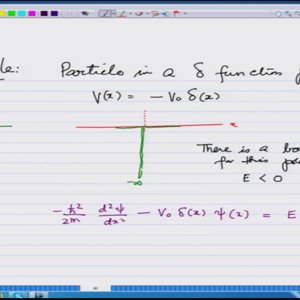
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 38: Solution of Schrodinger equation for a particle in delta functions
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 39: Solution of Schrodinger equation for a particle in a finite well
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 40: Solution of a one dimensional Schrodinger equation for bound states I
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 41: Solution of a one dimensional Schrodinger equation for bound states II
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 42: Reflection and transmission of particles across a potential barrier
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 43: Quantum-tunneling and its examples
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 44: Solution of the Schrodinger for free paticles and periodic boundary cond
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 45: Electrons in a metal : Density of states and Fermi energy
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 46: Schrodinger equation for particles in spherically symmetric potential
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 47: Angular momentum operator and its eigenfunctions
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 48: Equation for radial component of the wavefunction
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 49: Solution for radial component of the wavefunction for the hydrogen atom
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 50: Soln. for radial component of wavefunction for spherically sym potential
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 51: Bloch's theorem
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 52: Kroning-Penny model and energy bands
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
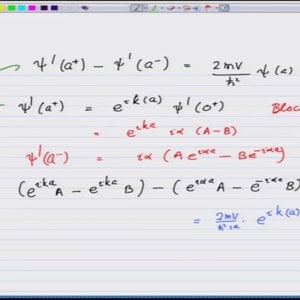
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 53: Kroning-Penny model with periodic Dirac delta function and energy bands
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 54: Discussion on Bands
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 55: Summary of the Course
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
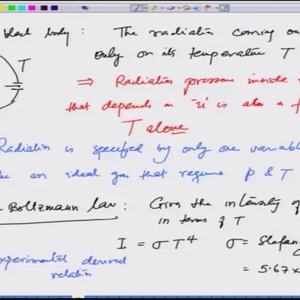
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 4: Black Body Radiation IV
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
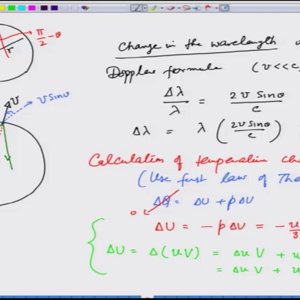
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 5: Black Body Radiation V
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Adiabatic Approximation in Hydrogen Atom
Homework Statement Assume that Planck's constant is not actually constant, but is a slowly varying function of time, $$\hbar \rightarrow \hbar (t)$$ with $$\hbar (t) = \hbar_0 e^{- \lambda t}$$ Where ##\hbar_0## is the value of ##\hbar## at ##t = 0##. Consider the Hydrogen atom in this case...- CDL
- Thread
- Replies: 6
- Forum: Advanced Physics Homework Help
-
B
A Nonlinear Schrodinger equation and linearity of Q.M.
Hello all, you may already know that Q.M. is a linear theory however there is something called nonlinear Sch. eq. for example Gross-Pitaevskii equation. How can such a thing exist considering that Q.M. is a strictly linear theory. Cheers.- Buddha_the_Scientist
- Thread
- Replies: 3
- Forum: Quantum Physics
-
B
I "Derivation" of the Schrödinger Equation
When reading a textbook I came across some reasoning about Schrödinger Equation. It started with the wave function $$\nabla^2\psi=k^2\psi$$ I am a bit lost at this point. Where does the right side of the equation come from? What should I review to fix that part of my knowledge?- BearY
- Thread
- Replies: 4
- Forum: Quantum Physics
-
I Does the Schrödinger equation link position and momentum?
I recently found this article about the dynamics of the wave function. It has some good simple illustrations and I found it valuable. But the author has a question himself, about understanding the Schrodinger equation. I wonder if anybody here could fill in the missing piece. The relevant part...- AuxPart
- Thread
- Replies: 3
- Forum: Quantum Physics
-
B
I Can the Schrodinger equation satisfy Laplace's equation?
The time-dependent Schrodinger equation is given by: ##-\frac{\hslash^{2}}{2m}\triangledown^{2}\psi+V\psi=i\hslash\frac{\partial }{\partial t}\psi## Obviously, there is a laplacian in the kinetic energy operator. So, I was wondering if the equation was rearranged as...- bb1414
- Thread
- Replies: 3
- Forum: Quantum Physics
-

I Hamiltonian in Schrödinger: necessarily total energy?
This is a basic question, so probably easy to answer. The following from Wikipedia seems pretty standard while describing the Schrödinger equation: "...and Ĥ is the Hamiltonian operator (which characterises the total energy of the system under consideration)." On the other hand, from page 100 of...- nomadreid
- Thread
- Replies: 2
- Forum: Quantum Physics
-
O
Step potential, continuous wave function proof
Homework Statement I am being asked to show that the wave function ψ and dψ/dx are continuous at every point of discontinuity for a step potential. I am asked to make use of the Heaviside step function in my proof, and to prove this explicitly and in detail. Homework Equations...- ope211
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
A What form of the Schrodinger equation do you use for intensity?
I am trying to see how I can use the schrodinger equation to quantify the changes in the intensity of light. My closest guess is using the time dependent pertubation theory- bluejay27
- Thread
- Replies: 1
- Forum: Quantum Physics
-
B
Schrodinger equation and boundary conditions
Hi at all, I'm tring to solve Schrodinger equation in spherically symmetry with these bondary conditions: ##\lim_{r \rightarrow 0} u(r)\ltimes r^{l+1}## ##\lim_{r \rightarrow 0} u'(r)\ltimes (l+1)r^{l}## For eigenvalues, the text I'm following says that I have to consider that the...- BRN
- Thread
- Replies: 2
- Forum: Calculus and Beyond Homework Help
-

I Question about the solution to the Harmonic wave equation
Hi, I have been looking in various text about how to find an admissible solution to the Schrödinger eqn in one dim. in the harmonic oscillator model. As in MQM, the solutions to this are said to be ##Ae^{ikx}+Be^{-ikx}##, which are then said to be not admissible. The book then goes straigtht to...- SemM
- Thread
- Replies: 8
- Forum: Quantum Physics
-
V
I I am interested in Schrodinger equation with tempor. element
I am interested in Schrodinger equation with the temporal element and the calculation of the probability when its depend on 3 variable coordinate and time? How to calculate it and norm it? Probability = (integral x,y,z...0 to infinity) * (integral t...0 to infinity)=1 ?- Viorel Popescu
- Thread
- Replies: 1
- Forum: Quantum Physics
-
B
Comp Sci [C++] Schrodinger equation solver and nuclear density
Hi everybody! I need to compute a C++ program for solve Schrodinger equation and calculate nuclear density. My nucleus is made up of only neutrons immersed in a potential of a harmonic oscillator. Schrodinger equation is: $$[-\frac{\hbar^2}{2m}\triangledown^2+V_{HO}(r)]\psi=E\psi$$ with...- BRN
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-

I How to identify admissible functions in QM by simple math?
Hi, in QM literature the inadmissible solutions to the Schrödinger eqn are often , if not always, quoted in the text as "inadmissible", because they are discontinuous, not-single valued, not square integrable and not infinitely differentiable. However in a discussion with Dr Du yesterday...- SemM
- Thread
- Replies: 2
- Forum: Quantum Physics
-
A
I Understanding Gauge Symmetry: A Review of the Schrödinger Equation
I have reviewed the various posts on gauge symmetry in particular this one which is now closed. In this post there is the following link:http://www.vttoth.com/CMS/physics-notes/124-the-principle-of-gauge-invariance. This is a good read. However, there is some clarification I need. The...- arlesterc
- Thread
- Replies: 3
- Forum: Quantum Physics
-
B
Schrodinger equation in cylindrical coordinates.
Hi guys! For nuclear case, I need to write an Schrodinger equation in cylindrical coordinates with an total potential formed by Woods-Saxon potential, spin-orbit potential and the Coulomb potential. Schrodinger equation can be written in this form: $$[-\frac{\hbar^2}{2m}(\frac{\partial...- BRN
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-

I Solving the Schrödinger eqn. by commutation of operators
Hi, I noticed that the raising and lowering operators:\begin{equation} A =\frac{1}{\sqrt{2}}\big(y+\frac{d}{dy}\big) \end{equation}\begin{equation} A^{\dagger}=\frac{1}{\sqrt{2}}\big(y-\frac{d}{dy}\big) \end{equation}can be used to solve the eqn HY = EY However I am curious about something...- SemM
- Thread
- Replies: 1
- Forum: Quantum Physics
-
S
A Solving the Schrödinger equation for free electrons
Dear all, sorry I made a new post similar to the previous post "Initial conditions..", however, a critical point was missed in the previous discussion: The initial conditions y(0)=1 and y'(0)=0 are fine and help in solving the Schrödinger equation, however, studying free electrons, the equation...- SeM
- Thread
- Replies: 2
- Forum: Quantum Physics
-
S
A What are typical initial conditions for the Schrödinger eq?
Hi, I am wondering if there exists some general initial conditions for solving the Schödinger eqn. for 1D free electrons ? Thanks!- SeM
- Thread
- Replies: 5
- Forum: Quantum Physics
-
J
B The deduction of Schrodinger equation, I'm stuck
Hi everyone! Please, I'm trying to understand the Schrodinger equation, and I've understood it this far, which which is a miracle, hehehe: (Laplacian)(psi) plus ((2phi)/h)^2.2m (E-V)(psi) I know that hbar = h/(2phi) But how that turns into (Laplacian)(psi)+2m/(hbar)^2.(E-V)(psi) My math...- Joao
- Thread
- Replies: 5
- Forum: Quantum Physics
-
V
B Schrödinger Equation for the fusion of Deuterium(2H) and a Proton(H)
In fact I am not sure if this is the right place to ask such a question but I'm going to ask anyways, just tell me if I am in the wrong place. So I doing a little experiment with the Schröndinger's equation, but the problem is I can't find a certain function. You all know the Schrödingers...- Viridun
- Thread
- Replies: 4
- Forum: Quantum Physics
-
D
Boundary conditions with l=0
The question is basically find the boundary conditions when ##l=0##, for energies minor than 0. Homework Equations $$V(r)=\begin{cases} & 0\text{ $r<a_0$}\\ &V_0\text{ $a_0<r<a_1$}\\ & 0\text{ $r>a_1$}\\ \end{cases} $$ $$...- Dario SLC
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
J
I Schrodinger equation and Heisenberg equation of motion
My question is that how does the Schrodinger equation arise from the Heisenberg equation of motion in the quantum field formalism. These are from Hatfield's book. So I'm having some difficulties to reproduce (2.36) by plugging (2.55) into (2.37) primarily because H is an integral...- Josh1079
- Thread
- Replies: 4
- Forum: Quantum Physics
-
W
Time-independent Schrödinger equation, normalizing
Homework Statement An electron coming from the left encounters/is trapped the following potential: -a<x<0; V=0 0<x<a; V=V0 infinity elsewhere the electron has energy V0 a)Write out the wave function b)normalize th wave function Homework EquationsThe Attempt at a Solution for -a<x<0...- WrongMan
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help