You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Schrodinger equation: Definition and 564 Discussions
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of the subject. The equation is named after Erwin Schrödinger, who postulated the equation in 1925, and published it in 1926, forming the basis for the work that resulted in his Nobel Prize in Physics in 1933.Conceptually, the Schrödinger equation is the quantum counterpart of Newton's second law in classical mechanics. Given a set of known initial conditions, Newton's second law makes a mathematical prediction as to what path a given physical system will take over time. The Schrödinger equation gives the evolution over time of a wave function, the quantum-mechanical characterization of an isolated physical system. The equation can be derived from the fact that the time-evolution operator must be unitary, and must therefore be generated by the exponential of a self-adjoint operator, which is the quantum Hamiltonian.
The Schrödinger equation is not the only way to study quantum mechanical systems and make predictions. The other formulations of quantum mechanics include matrix mechanics, introduced by Werner Heisenberg, and the path integral formulation, developed chiefly by Richard Feynman. Paul Dirac incorporated matrix mechanics and the Schrödinger equation into a single formulation. When these approaches are compared, the use of the Schrödinger equation is sometimes called "wave mechanics".
View More On Wikipedia.org
The Schrödinger equation is not the only way to study quantum mechanical systems and make predictions. The other formulations of quantum mechanics include matrix mechanics, introduced by Werner Heisenberg, and the path integral formulation, developed chiefly by Richard Feynman. Paul Dirac incorporated matrix mechanics and the Schrödinger equation into a single formulation. When these approaches are compared, the use of the Schrödinger equation is sometimes called "wave mechanics".
View More On Wikipedia.org
-
M
Non trivial solution to Schrödinger equation for 1-D infinite well
Hello, I am trying to find the solution of Schrödinger equation on matlab. However, when I apply boundary conditions, MATLAB only gives me the solution with both coefficients 0. I want to find the solution : Asin(n*pi*x/L) You can see my code below. Could you please tell me where is my mistake...- MrMuscle
- Thread
- Replies: 3
- Forum: MATLAB, Maple, Mathematica, LaTeX
-
F
I Solving the Quantum Mechanics of a Hydrogen Atom
Hello, I have a little problem understanding the quantum mechanics of a hydrogen atom. Im troubled with the following question: before i measure the state of a (simplified: without fine-, hyperfinestructure) hydrogen atom, which is the right probability density of finding the electron? is it...- frustrationboltzmann
- Thread
- Replies: 3
- Forum: Quantum Physics
-
A
A Do we need stochasticity in a discrete spacetime?
Suppose that the spacetime is discrete, with only certain positions being possible for any particle. In this case, the probability distributions of particles have nonzero values at the points on which the wavefunction is defined. Do we need randomness in the transitions of particles in such a...- Ali Lavasani
- Thread
- Replies: 2
- Forum: Quantum Physics
-
Z
Show that this Equation Satisfies the Schrodinger Equation
I apologize for the bad formatting: To start off, I'm trying to use the Schrodinger Equation in the form: (ħ/2m) d^2Ψ(x,t)/dx^2+V(x,t)Ψ(x,t)=EΨ(x,t) I couldn't remember if I need to also take the partial derivative with respect to T as well, but I started off with just X. I plugged in my...- Zinggy
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-
G
I General solution to the Time-independent Schrödinger equation?
Has anyone formulated a general solution to the time-independent Schrödinger equation in terms of the potential function V(r), and if so, what is it? For any type of V(r). So, instead of a differential equation, a direct relationship between the wavefunction and the potential.- greswd
- Thread
- Replies: 8
- Forum: Quantum Physics
-

A Schrödinger Evolution of Self-Gravitating Disks
This paper was recently published in the Monthly Notices of the Royal Astronomical Society. Batygin 2018, Schrödinger Evolution of Self-Gravitating Disks I am posting this in here, but I am actually more interested in the implications of looking at this the other way around: namely, from a...- Auto-Didact
- Thread
- Replies: 1
- Forum: Astronomy and Astrophysics
-

Finding the 10 lowest energy levels
Homework Statement Homework Equations The Attempt at a Solution I understand the equation, and I understand the concept. My question is this: What is the best way to go about solving this problem? My line of reasoning concludes that the fourth lowest energy level is E211. However, the...- Mason Smith
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-

I Novel Schrodinger equation examples for 1D
I've been studying the 1D schrodinger equation, and getting a feel for solutions in the harmonic oscillator, or potentials of inverse radius (atomic/hydrogen), and many versions of stair-step/ square potentials (square wells.) But, I've noticed that there are very few exact 1D potentials in the...- learn.steadfast
- Thread
- Replies: 5
- Forum: Quantum Physics
-

MATLAB Code: Stationary Schrodinger EQ, E Spec, Eigenvalues
Hello everyone, For weeks I have been struggling with this quantum mechanics homework involving writing a code to determine the energy spectrum and eigenvalues for the stationary Schrodinger equation for the harmonic oscillator. I can't find any resources anywhere. If anyone could help me get...- Baynie
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-

I About the Schrödinger equation
Hi everybody, For solving the time dependent Schrödinger equation ##H|\psi(t) \rangle = i\hbar \frac{\partial}{\partial t}|\psi (t)\rangle##, I read in quantum mechanics books the assumption about the solution ##|\psi(t) \rangle## which is made of a linear combination of a complete set of the...- Konte
- Thread
- Replies: 19
- Forum: Quantum Physics
-
S
Prove that ##\psi## is a solution to Schrödinger equation
Homework Statement For a wavefunction ##\psi##, the variance of the Hamiltonian operator ##\hat{H}## is defined as: $$\sigma^2 = \big \langle \psi \mid (\hat{H} - \langle\hat{H}\rangle)^2 \psi \big\rangle$$ I want to prove that if ##\sigma^2 = 0##, then ##\psi## is a solution to the...- Sofie RK
- Thread
- Replies: 9
- Forum: Introductory Physics Homework Help
-
D
Finding Stationary Wavefunction with a Line Potential
Homework Statement A particle of mass m in one dimension has a potential: $$V(x) = \begin{cases} V_0 & x > 0 \\ 0 & x \leq 0 \end{cases} $$ Find ##\psi(x)## for energies ##0 < E < V_0##, with parameters $$k^2 = \frac{2mE}{\hbar^2}$$ and $$\kappa^2 = \frac{2m(V_0 - E)}{\hbar^2}$$...- doggydan42
- Thread
- Replies: 9
- Forum: Advanced Physics Homework Help
-

I Why is separation constant l(l+1) instead of +-l^2?
While separating variables in the Schrodinger Equation for hydrogen atom, why are we taking separation constant to be l(l+1) instead of just l^2 or -l^2, is it just to make the angular equation in the form of Associated Legendre Equation or is there a deeper meaning to it?- Rupul Chandna
- Thread
- Replies: 2
- Forum: Quantum Physics
-

Time Derivative of Expectation Value of Position
Homework Statement I want to prove that ##\frac{\partial \langle x \rangle}{\partial t} = \frac{\langle p_x \rangle}{m}##. Homework Equations $$i\hbar \frac{\partial \Psi}{\partial t} = -\frac{\hbar^2}{2m} \frac{\partial^2 \Psi}{\partial x^2} + V \Psi$$ The Attempt at a Solution [/B] So...- Matt Chu
- Thread
- Replies: 8
- Forum: Advanced Physics Homework Help
-
P
Finding the quantized energies of a particle
Homework Statement Okay, so the question I'm trying to solve is to find the quantized energies for a particle in the potential: $$V(x)=V_0 \left ( \frac{b}{x}-\frac{x}{b} \right )^2$$ for some constant b. The Attempt at a Solution I am following along with the derivation of the quantized...- physics148
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
J
Quantum I need a book to solve Schrodinger's eqn numerically
I have found this one that looks perfect: https://www.amazon.com/dp/331999929X/?tag=pfamazon01-20 THe problem is that it has not been published yet :( , but I can't believe there is no other book on the subject. What I want is to solve numerically the Schrodinger equation with no special...- jonjacson
- Thread
- Replies: 7
- Forum: Science and Math Textbooks
-

Discrepancies between numerical and analytical solutions
The analytical solutions are: \begin{equation} \psi(x) = \begin{cases} Ce^{\alpha x}, \text{if } x < -\frac{L}{2}\\ Asin(kx) + Bcos(kx), \text{if } -\frac{L}{2} \leq x \leq \frac{L}{2}\\ Fe^{-\alpha x} , \text{if } x > \frac{L}{2} \end{cases} \end{equation}...- Ziezi
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-
A
I Finite square well bound states
Let's suppose I have a finite potential well: $$ V(x)= \begin{cases} \infty,\quad x<0\\ 0,\quad 0<x<a\\ V_o,\quad x>a. \end{cases} $$ I solved the time-independent Schrodinger equation for each region and after applying the continuity conditions of ##\Psi## and its derivative I ended up with...- andrewtz98
- Thread
- Replies: 1
- Forum: Quantum Physics
-
K
I Difference between Schrodinger's equation and wave function?
Is there a difference between Schrodinger's equation and the wave function? In the beginning of the second edition by David J. Griffiths he compares the classical F(x,t) and Schrodinger's equation and I am having trouble understanding the connection.- Kiley
- Thread
- Replies: 2
- Forum: Quantum Physics
-

Dirac hydrogen atom vs spin symmetry
Homework Statement Exact spin symmetry in the Dirac equation occurs when there is both a scalar and a vector potential, and they are equal to each other. What physical effect is absent in this case, that does exist in the Dirac solution for the hydrogen atom (vector potential = Coulomb and...- It's me
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
X
Energy of Hydrogen 1s using simplified Schrodinger equation
Homework Statement [/B] The Hamiltonian and wavefunction for the ground state of the hydrogen atom H(1s1) are given, in atomic units, as ## \hat {H} = - \frac{1}{2} \nabla^2 - \frac {1}{r} ## and ## \phi(1s) = \sqrt {\frac {1}{\pi }} e^{-r} ## . Using the radial portion of the Laplacian in the...- Xilus1
- Thread
- Replies: 4
- Forum: Calculus and Beyond Homework Help
-

Normalization of wave function
Homework Statement I have the wave function Ae^(ikx)*cos(pix/L) defined at -L/2 <= x <= L/2. and 0 for all other x. The question is: A proton is in a time-independent one-dimensional potential well.What is the probability that the proton is located between x = − L/4 and x = L/4 ? Homework...- Safder Aree
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 1: Black Body Radiation I
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 2: Black Body Radiation II
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 3: Black Body Radiation III
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 6: Black Body Radiation VI
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 7: Black Body Radiation VII
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
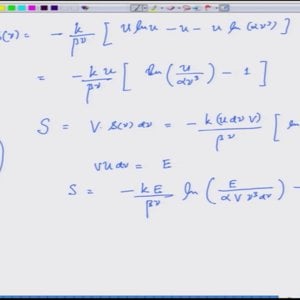
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 8: Radiation as a collection of particles called photons
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 9: Quantum Hypothesis and specific heat of solids
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 10: Bohr's Model of hydrogen spectrum
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 11: Wilson Sommerfeld quantum condition I
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 12: Wilson Sommerfeld quantum condition II
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 13: Wilson Sommerfeld quantum condition III
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 14: Quantum conditions and atomic structure
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 15: Eienstien's A and B coefficients
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 16: Stimulated emission and amplification of light in a LASER
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-
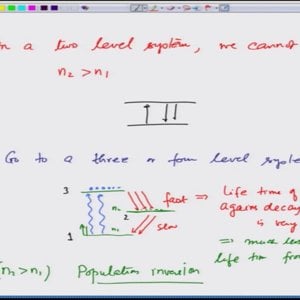
Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 17: Brief description of a LASER
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 18: Introduction to the correspondence principle
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 19: General nature of the correspondence principle
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 20: Selection rules (for transitions) through the correspondence principle
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 21: Applications of the correspondence principle
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 22: Heisenberg's formulations of quantum mechanics I
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 23: Heisenberg's formulations of quantum mechanics II
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 24: Heisenberg's formulations of quantum mechanics III
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 25: Brief introduction to matrix mechanics
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 26: Introduction to waves and wave equation
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 27: Sationary waves eigen values and eigen functions
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 28: Matter waves and their experimental detection
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 29: Representing a moving particle by a wave packet
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum
-

Introductory Quantum Mechanics with Prof. Manoj Harbola (NPTEL):- Lecture 30: Stationary-state Schrodinger equation
All copyright reserved to Prof. Harbola and NPTEL, Govt. of India. Duplication punishable offence. Course Website: http://www.nptel.ac.in/courses/115104096/- Wrichik Basu
- Media item
- black body radiation quantum basics schrodinger equation
- Comments: 0
- Category: Quantum