- #1
guyvsdcsniper
- 264
- 37
- Homework Statement
- Convert Voltage into pressure
- Relevant Equations
- y=mx+b
I am measuring the index of refraction of air using a Michelson interferometer.
This set up includes a gas chamber that is placed on of the paths of source laser after it passes through a beam splitter. The gas chamber is pumped to vacuum, and then air is allowed to slowly enter the gas chamber. The introduction of air is enough to slow down the laser passing through it to cause a phase shift. The two beams of the laser then recombine at the beam splitter and emit a sweeping interference pattern onto a photodetector, that measures the the change in interference pattern as voltage difference.
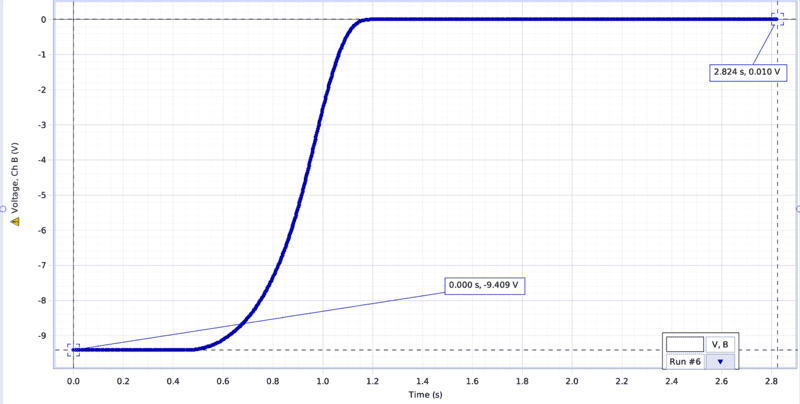
The change in pressure in the gas chamber is measured via voltage difference via a pressure gauge. Vacuum is approximately -9.409 V and atmosphere is approximately .010V.
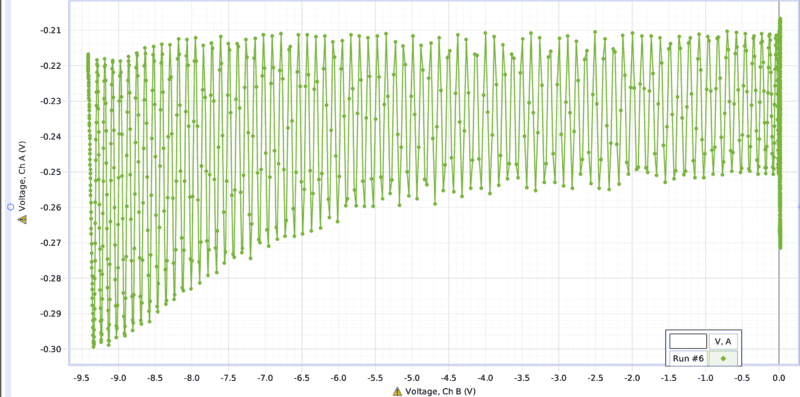
My goal is to take the data from one of these runs and plot it as Intensity of the photo detector (Ch A) vs the pressure of the pressure gauge(Ch B.).
I am fine with leaving the intensity in terms of voltage, but leaving my x axis as pressure in terms of voltage doesnt seem right to me.
So I plotted the pressure gauge voltage with respect to time, which can be seen in the photo below, plotted in blue. I found the slope to be 3.34 and my y intercept be -9.409. So I have the equation y = 3.34x-9.409, where x is a certain time in the run. This should give me the voltage at any instance of time. I tried plugging in certain time values but the voltage values I receive dont match whats actually on the graph.
If this above equation did work, I thought that if 1atm = 9.409 Volts, I can take the ratio of 1atm/y and convert volts to atm.
But this doesn't seem to work for me.
This set up includes a gas chamber that is placed on of the paths of source laser after it passes through a beam splitter. The gas chamber is pumped to vacuum, and then air is allowed to slowly enter the gas chamber. The introduction of air is enough to slow down the laser passing through it to cause a phase shift. The two beams of the laser then recombine at the beam splitter and emit a sweeping interference pattern onto a photodetector, that measures the the change in interference pattern as voltage difference.
The change in pressure in the gas chamber is measured via voltage difference via a pressure gauge. Vacuum is approximately -9.409 V and atmosphere is approximately .010V.
My goal is to take the data from one of these runs and plot it as Intensity of the photo detector (Ch A) vs the pressure of the pressure gauge(Ch B.).
I am fine with leaving the intensity in terms of voltage, but leaving my x axis as pressure in terms of voltage doesnt seem right to me.
So I plotted the pressure gauge voltage with respect to time, which can be seen in the photo below, plotted in blue. I found the slope to be 3.34 and my y intercept be -9.409. So I have the equation y = 3.34x-9.409, where x is a certain time in the run. This should give me the voltage at any instance of time. I tried plugging in certain time values but the voltage values I receive dont match whats actually on the graph.
If this above equation did work, I thought that if 1atm = 9.409 Volts, I can take the ratio of 1atm/y and convert volts to atm.
But this doesn't seem to work for me.