- #1
TGH904
Hello all,
I have a question about the electrical engineering necessary to design an experiment in which one would test various electrical parameters, such as high voltages, low voltages, low currents, varying signals (direct current, square waves, etc) on biofilms, and bacteria.
I have asked a similar question on the Arduino forums asking how I can implement an Arduino in a power supply design to control the electrical parameters, but now I am wondering more about the bacteria as part of the circuit.
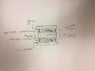
Essentially the bacteria will be grown, and stored on a petri dish, or a biofilm grown on a 'coupon', and with some power supply system, providing measurable, and controllable voltage, current, and signals will be directly applied to these substances.
My questions are:
1) Can someone point me in the right direction of a power supply that will provide for such control, or the schematic for building my own? (There may be more ways to tackle this question I am open to suggestions)
2) What kind of engineering equations in the electrical world will I need to understand, and know for evaluating my experiment? (In the electrical engineering world, not the microbiology evaluation techniques)
3) What impact will the bacteria have as a circuit element?*
*For the last question I am not sure if it is best to ask a microbiologist as they would know more about the fundamental properties of bacteria/biofilms, yet they may lack the basic, or advanced understanding of electrical engineering fundamentals and how to apply those concepts.
Does this make sense? I will provide links to various articles where other researchers have conducted their own experiments using electrical stimulation on bacteria/biofilms, so that anyone interested in this project/topic may review them. As well as a link to the Arduino forum where I have an on going discussion there as well.
I appreciate your time, and help. Thank you very much and I look forward to hearing back from you.
https://forum.arduino.cc/index.php?topic=488615.0
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.548.8043&rep=rep1&type=pdf
http://www.rehab.research.va.gov/jour/04/41/2/pdf/Merriman.pdf
http://aac.asm.org/content/52/10/3517.full
I have a question about the electrical engineering necessary to design an experiment in which one would test various electrical parameters, such as high voltages, low voltages, low currents, varying signals (direct current, square waves, etc) on biofilms, and bacteria.
I have asked a similar question on the Arduino forums asking how I can implement an Arduino in a power supply design to control the electrical parameters, but now I am wondering more about the bacteria as part of the circuit.
Essentially the bacteria will be grown, and stored on a petri dish, or a biofilm grown on a 'coupon', and with some power supply system, providing measurable, and controllable voltage, current, and signals will be directly applied to these substances.
My questions are:
1) Can someone point me in the right direction of a power supply that will provide for such control, or the schematic for building my own? (There may be more ways to tackle this question I am open to suggestions)
2) What kind of engineering equations in the electrical world will I need to understand, and know for evaluating my experiment? (In the electrical engineering world, not the microbiology evaluation techniques)
3) What impact will the bacteria have as a circuit element?*
*For the last question I am not sure if it is best to ask a microbiologist as they would know more about the fundamental properties of bacteria/biofilms, yet they may lack the basic, or advanced understanding of electrical engineering fundamentals and how to apply those concepts.
Does this make sense? I will provide links to various articles where other researchers have conducted their own experiments using electrical stimulation on bacteria/biofilms, so that anyone interested in this project/topic may review them. As well as a link to the Arduino forum where I have an on going discussion there as well.
I appreciate your time, and help. Thank you very much and I look forward to hearing back from you.
https://forum.arduino.cc/index.php?topic=488615.0
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.548.8043&rep=rep1&type=pdf
http://www.rehab.research.va.gov/jour/04/41/2/pdf/Merriman.pdf
http://aac.asm.org/content/52/10/3517.full