- #1
Ryaners
- 50
- 2
I'm trying to solve one of the problems in my textbook (Atkins Physical Chemistry) and I just can't get it out. Here's the problem:
"The data below apply to the formation of urea from ammonium cyanate. Initially 22.9g of ammonium cyanate was dissolved in enough water to prepare 1.00 dm3 of solution. Determine the order of the reaction , the rate constant, and the mass of ammonium cyanate left after 300min.
t / min: 0 20.0 50.0 65.0 150
mass / g: 0 7.0 12.1 13.8 17.7 "
From the question I know that the concentration will be equal in magnitude to the mass / amount in moles as the volume is 1 dm3. So based on this assumption I calculated the following from the given data (taking the molar mass of urea to be 60.06g):
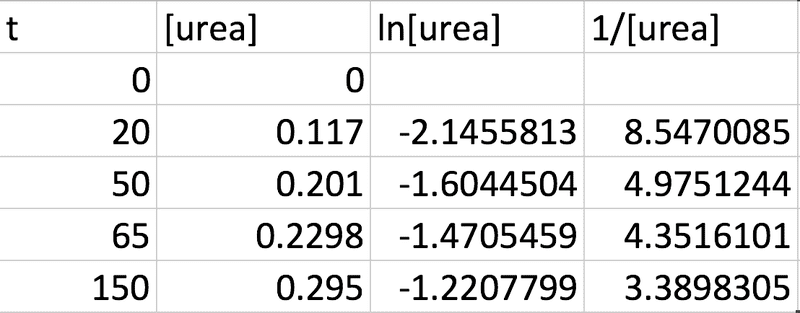
This is based on the idea that the integrated rate laws for unimolecular reactions are:
0th: [urea]t = [urea]0 - kt
1st: ln[urea]t = ln[urea]0 -kt
2nd: 1 / [urea]t = 1 / [urea]0 + kt
So, in each case, the relationship between some particular expression of [urea] and t is linear, which makes sense to me, and the slope of the graph will be equal to -k. (Or +k in this case, I think, because it's the product concentration I'm given.) BUT when I graph each of the sets of data against time, none of them are linear:
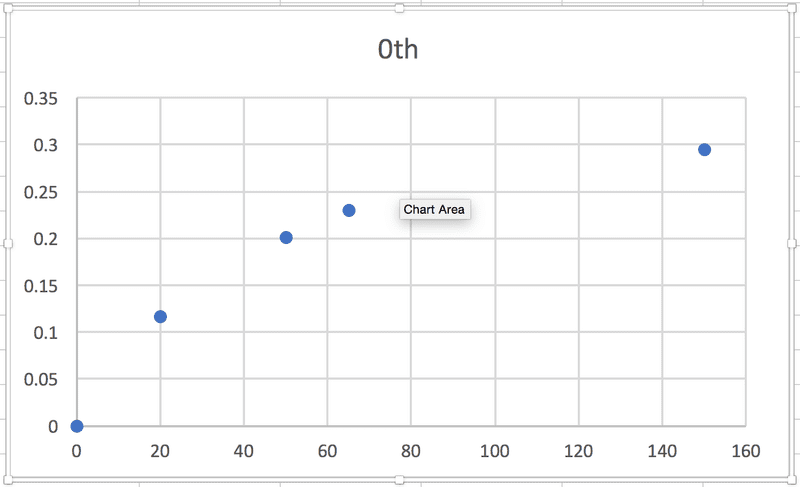
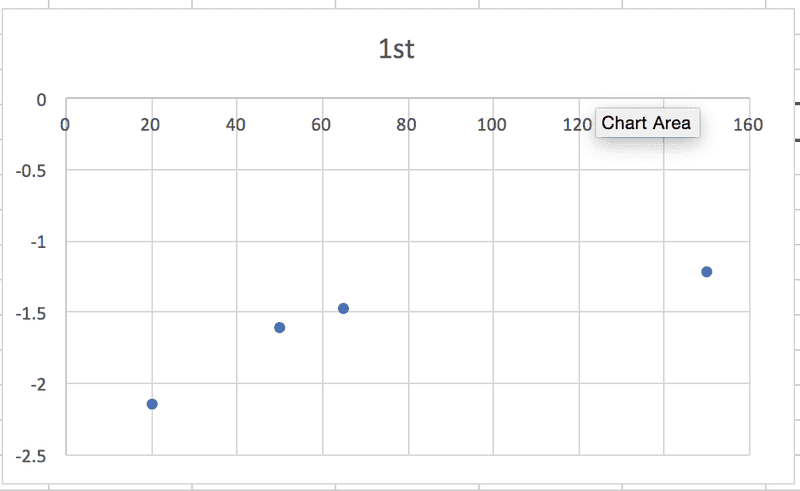
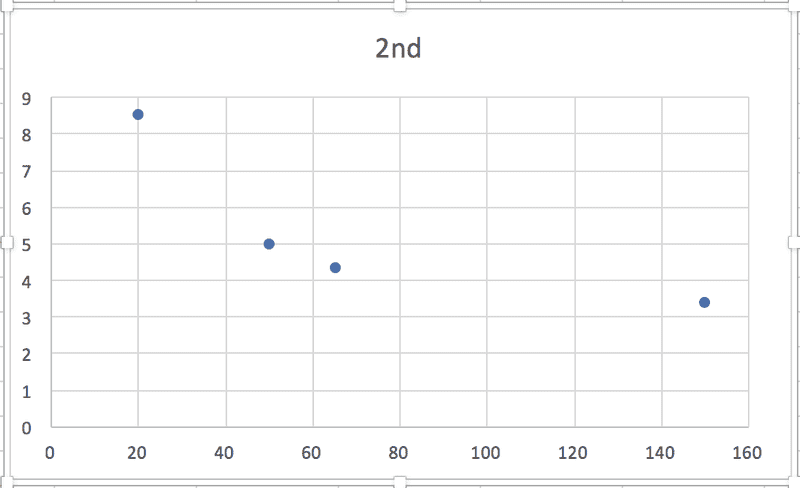
According to the textbook, the reaction is 2nd order. Where am I going wrong?! Thanks in advance for any help.
"The data below apply to the formation of urea from ammonium cyanate. Initially 22.9g of ammonium cyanate was dissolved in enough water to prepare 1.00 dm3 of solution. Determine the order of the reaction , the rate constant, and the mass of ammonium cyanate left after 300min.
t / min: 0 20.0 50.0 65.0 150
mass / g: 0 7.0 12.1 13.8 17.7 "
From the question I know that the concentration will be equal in magnitude to the mass / amount in moles as the volume is 1 dm3. So based on this assumption I calculated the following from the given data (taking the molar mass of urea to be 60.06g):
This is based on the idea that the integrated rate laws for unimolecular reactions are:
0th: [urea]t = [urea]0 - kt
1st: ln[urea]t = ln[urea]0 -kt
2nd: 1 / [urea]t = 1 / [urea]0 + kt
So, in each case, the relationship between some particular expression of [urea] and t is linear, which makes sense to me, and the slope of the graph will be equal to -k. (Or +k in this case, I think, because it's the product concentration I'm given.) BUT when I graph each of the sets of data against time, none of them are linear:
According to the textbook, the reaction is 2nd order. Where am I going wrong?! Thanks in advance for any help.

