- #1
Martin Harris
- 103
- 6
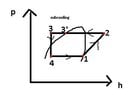
The heat pump comprises of the 4 components: evaporator, compressor, condenser and expansion valve.
Thermal power required to heat the building: 12.1 kW at condensing temperature tc = 44.3 deg C
For the evaporator: vaporizing temperature tv = -7 deg C
Subcooling temperature for the heat pump Δ tsc = 5 deg C
adiabatic coefficient: k = 1.16
vaporising pressure: pv =t(tv) =paspiration =4bar
condensing pressure: pc = t(tc) = pdischarge =17bar
vapor density: ρv=17.0 kg/m3
specific volume for aspiration vaspiration = 1/ρv = 1/17kg/m3 = 0.058823529 m3/kg
internal compressor efficiency: ηi=0.84
h1= hv(tv) = 402.56 kJ/kg
h3 =hl(tsc,pc) =248.6 kJ/kg
Now I tried finding out the following:
a)Thermal power Q0 at the evaporator
b)Compressor power
c)efficiency of the heat pump (both COP and refrigeration cycle efficiency ε )
a)Q0=q0*mrf, where q0 = specific heat load, and mrf=mass floware for the refrigerant
q0 = h1-h4 = h1-h3; because h3=h4
so q0 = 402.56 kJ/kg - 248.6 kJ/kg => q0 = 153.96kJ/kg
compressor work: compressor_work = (k/((k-1)*ηi))*paspiration*vaspiration*((pdischarge/paspiration)^((k-1)/k)-1)*100
compressor_work = 44.85735668 kJ/kg
enthaply in the 2nd point: h2 = h1+compressor_work = 402.56 kJ/kg+44.85735668 kJ/kg
h2 = 447.4173567 kJ/kg
specific heat load at the condenser: qc = h2-h3 = 447.4173567 kJ/kg - 248.6 kJ/kg
qc =198.8173567 kJ/kg
so: mrf = Qc/qc = 12.1 kW/198.8173567 kJ/kg
mrf = 0.060859878 kg/s (refrigerant mass flowrate)
Thermal power Q0 at the evaporator: Q0=q0*mrf
Q0 = 153.96kJ/kg*0.060859878 kg/s
Q0 = 9.369986761 kW
b)Compressor_power = (mass_flowrate_refrigerant * compressor_work) / (ηmotor*ηelectrical)
Problem arises here because I was given just the internal efficiency ηi=0.84, but the formula uses the efficiency of the motor ηmotor and the electrical efficiency ηelectrical...so how can I calculate this? (as I don't have the ηmotor*ηelectrical), I don't want to guess though...
c)COP =qc/work_compressor = 198.8173567 kJ/kg / 44.85735668 kJ/kg
COP = 4.432
ε = heat load evaporator/work done = COP - 1 = 3.432
What do you think of the calculations, do they make any sense? I'd really appreciate a peer review.
My actual question is, how can I calculate the compressor power at b) as I don't have those 2 efficiencies (ηmotor and ηelectrical), but just the ηi which is the internal efficiency of the compressor?
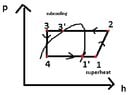
Thermal power required to heat the building: 12.1 kW at condensing temperature tc = 44.3 deg C
For the evaporator: vaporizing temperature tv = -7 deg C
Subcooling temperature for the heat pump Δ tsc = 5 deg C
adiabatic coefficient: k = 1.16
vaporising pressure: pv =t(tv) =paspiration =4bar
condensing pressure: pc = t(tc) = pdischarge =17bar
vapor density: ρv=17.0 kg/m3
specific volume for aspiration vaspiration = 1/ρv = 1/17kg/m3 = 0.058823529 m3/kg
internal compressor efficiency: ηi=0.84
h1= hv(tv) = 402.56 kJ/kg
h3 =hl(tsc,pc) =248.6 kJ/kg
Now I tried finding out the following:
a)Thermal power Q0 at the evaporator
b)Compressor power
c)efficiency of the heat pump (both COP and refrigeration cycle efficiency ε )
a)Q0=q0*mrf, where q0 = specific heat load, and mrf=mass floware for the refrigerant
q0 = h1-h4 = h1-h3; because h3=h4
so q0 = 402.56 kJ/kg - 248.6 kJ/kg => q0 = 153.96kJ/kg
compressor work: compressor_work = (k/((k-1)*ηi))*paspiration*vaspiration*((pdischarge/paspiration)^((k-1)/k)-1)*100
compressor_work = 44.85735668 kJ/kg
enthaply in the 2nd point: h2 = h1+compressor_work = 402.56 kJ/kg+44.85735668 kJ/kg
h2 = 447.4173567 kJ/kg
specific heat load at the condenser: qc = h2-h3 = 447.4173567 kJ/kg - 248.6 kJ/kg
qc =198.8173567 kJ/kg
so: mrf = Qc/qc = 12.1 kW/198.8173567 kJ/kg
mrf = 0.060859878 kg/s (refrigerant mass flowrate)
Thermal power Q0 at the evaporator: Q0=q0*mrf
Q0 = 153.96kJ/kg*0.060859878 kg/s
Q0 = 9.369986761 kW
b)Compressor_power = (mass_flowrate_refrigerant * compressor_work) / (ηmotor*ηelectrical)
Problem arises here because I was given just the internal efficiency ηi=0.84, but the formula uses the efficiency of the motor ηmotor and the electrical efficiency ηelectrical...so how can I calculate this? (as I don't have the ηmotor*ηelectrical), I don't want to guess though...
c)COP =qc/work_compressor = 198.8173567 kJ/kg / 44.85735668 kJ/kg
COP = 4.432
ε = heat load evaporator/work done = COP - 1 = 3.432
What do you think of the calculations, do they make any sense? I'd really appreciate a peer review.
My actual question is, how can I calculate the compressor power at b) as I don't have those 2 efficiencies (ηmotor and ηelectrical), but just the ηi which is the internal efficiency of the compressor?