- #1
etotheipi
It is given that the solution is ideal, i.e. that we can take ##\gamma_A = 1##.
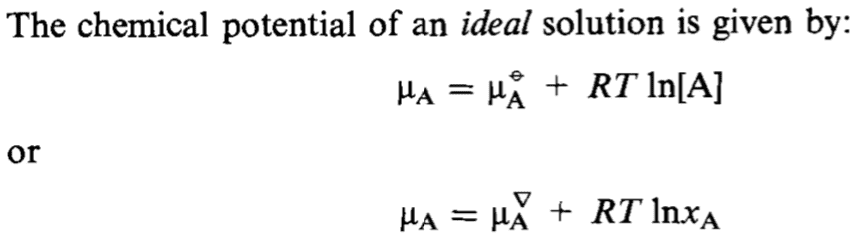
I wondered what that small triangle signifies in the second definition? Thanks!
I wondered what that small triangle signifies in the second definition? Thanks!