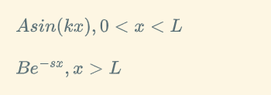- #1
drop_out_kid
- 34
- 2
- Homework Statement
- For a well left side is infinite potential and right side is finite potential V0 , and the energy is constant and less than V0(also constant). Now get the wave function , get the equation for eigen energy(the energy allowed) and the conditions for no solution
- Relevant Equations
- $$-\frac{\hbar^2}{2m} \frac{\partial^2}{\partial x^2} \Phi(x)+V(x)\Phi(x)=0$$
So I have come up with my solution(attempt) which is:
where
(
$$\psi_ 1 \triangleq Asin(kx),0<x<L$$
$$\psi_ 2 \triangleq Be^{-sx},x>L$$
$$k \triangleq \sqrt{\frac{2mE}{\hbar^2}} $$
$$s \triangleq \sqrt{\frac{2m(V-E)}{\hbar^2}} $$)
But this has a serious problem about boundary: I think I should use that
But this is what I get:
So:
1. How can I get this normalisation in a easy way?
2. Can I use this for part (b) to solve eigen energy?Thought for hours , assignment is going to due (