- #1
MB613d
- 13
- 0
Homework Statement
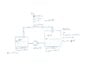
Two half with a fluid filled vessels are connected through two pipes in a closed system. The bottom pipe pumps the fluid from vessel 1 to vessel 2 at a rate of 300m³/h. The fluid pushes the air of vessel 2 through the top pipe into vessel 1. What is the pressure within the vessels? Drawing attached.
Data
Pipe:
DN = 150 mm
l = 20 m
Zeta = 15
Vessel 1:
V1 = 5.000 m³
V2_Gas = 2.500 m³
Vessel 2:
V2 = 10.000 m³
V2_Gas = 5.000m³
Overall:
p0 = pressure before the pump starts = athmospheric = 1,013 bar
T = const = 273,15 K
V1 = const
V2 = const
EDIT: In the drawing lambda should be zeta
Homework Equations
p*V=n*R*T ?
The Attempt at a Solution
I calculated the pressure drop within the pipe to be dp = 2,5 mbar.
Now I assume that when the pump starts the pressure p2 in vessel 2 will equal the pressure loss within the pipe plus p0. I use the ideal gas law to calculate the mass of the air within vessel 2 at the pressure p0 and p0+dp. I calculate the difference between the mass at p0 and p0+dp and substract it from the mass in vessel 1. Now I calculate the pressure in vessel 1 with the calculated mass.
I guess it's not right. I have spent a long time in the library to find a similar problem but couldn't find anything. I would really appreciate your help. Thank you all in advance. PS: Please excuse my english.
Attachments
Last edited:
 ##\qquad## !
##\qquad## !