- #1
bomba923
- 763
- 0
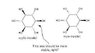
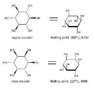
Scyllo-inositol should be more stable than myo-inositol, right? (unless I'm missing a certain detail)
But in nearly all literature on inositols, it is always myo-inositol that is primarily focused on. Scyllo-inositol is said to exist in "trace amounts" along with other inositols, while myo-inositol is the most common of all inositols. Also, Wikipedia does not mention scyllo-inositol (only myo-inositol) (see). In general, it seems scyllo-inositol (which I think is the most stable of all inositols) is barely mentioned in inositol literature.
Perhaps myo-inositol is more "useful" nutritionally and otherwise than scyllo-inositol.
But is also more structurally stable than scyllo-inositol??
(Edit: for those who don't know, inositol (C6H12O6) is a structural isomer of glucose; its IUPAC
name would be 1,2,3,4,5,6-cyclohexanehexol. See the attached image for more information)
But in nearly all literature on inositols, it is always myo-inositol that is primarily focused on. Scyllo-inositol is said to exist in "trace amounts" along with other inositols, while myo-inositol is the most common of all inositols. Also, Wikipedia does not mention scyllo-inositol (only myo-inositol) (see). In general, it seems scyllo-inositol (which I think is the most stable of all inositols) is barely mentioned in inositol literature.
Perhaps myo-inositol is more "useful" nutritionally and otherwise than scyllo-inositol.
But is also more structurally stable than scyllo-inositol??
(Edit: for those who don't know, inositol (C6H12O6) is a structural isomer of glucose; its IUPAC
name would be 1,2,3,4,5,6-cyclohexanehexol. See the attached image for more information)
Attachments
Last edited:


 ).
).