Here's a link to the (freely available) scientific paper describing the antiviral strategy:
Rider TH, Zook CE, Boettcher TL, Wick ST, Pancoast JS,
et al. (2011) Broad-Spectrum Antiviral Therapeutics.
PLoS ONE 6(7): e22572.
doi:10.1371/journal.pone.0022572
My analysis of the paper:
Many viruses, during their replication phase, create fairly long double-stranded RNA (dsRNA) molecules. For example, the influenza virus copies its genome through a dsRNA intermediate. In contrast, mammalian cells usually do not produce many long (>~23nt) dsRNA molecules. Therefore, the presence of long dsRNAs inside the cell are a good indicator of viral infection. Indeed, many cellular antiviral defenses are based on the recognition of dsRNA.
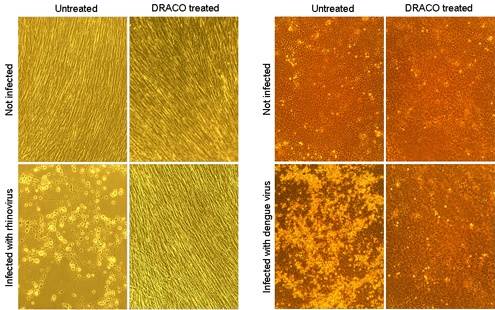
In the paper, the authors create a class of protein-based agent (called DRACOs) that senses double-stranded RNA (dsRNA), and when the protein senses dsRNA, it tells the cell to undergo apoptosis, programmed cell death, in order to kill the cell before the virus can copy itself. These DRACOs are able to enter cells in culture and protect the culture from infection by a variety of viruses (the study tested 15 viruses from a variety of virus families). The DRACOs also do not seem to be harmful to uninfected cells (the study tested 11 lines of cultured mammalian cell).
These DRACO agents do seem like they could show some promise, although more research need to be done to determine how effective they will work in patients (versus just in cultured cells). Because the DRACO agents are proteins, they are very susceptible to degradation by the body. I have doubts that systemic application of these proteins into a patient would deliver enough of them to the infected tissue to help control the infection.
Because the DRACOs are engineered from components of the cell already used to combat viral infections (dsRNA sensing proteins and proteins that induce apoptosis), there is some concern that some of the mechanisms that viruses have evolved to evade the cellular antiviral defenses may also be effective against the DRACOs. For example, some viruses have evolved ways to prevent apoptosis (cytotoxic T-cells help fight infection by telling infected cells to undergo apoptosis). However, these DRACOs activate the apoptotic pathway fairly directly and may be able to get around some of the tricks viruses use to inhibit apoptosis. A larger concern are the tricks that viruses have evolved to mask their dsRNA from detection in the cell.
Finally, although the authors show efficacy against a broad spectrum of viruses, not all viruses produce long dsRNAs that can be recognized by the DRACOs. Retroviruses like HIV do not contain long stretches of dsRNA that would activate the DRACOs, so the DRACOs would likely be ineffective against this class of virus.