Kolin101
- 4
- 0
- Homework Statement
- An insulated tank of volume 12 m3 and with a valve was filled with perfect triatomic gas at pressure 10 bar. Initially the valve was closed. A paddle wheel was mounted inside the tank and was run by 20 kW engine. The paddle wheel was turn on and at the same time the exit valve was opened. The gas temperature was constant trough the whole process (due to very slow flow) and equal to 300 K.
Calculate the time needed to decrease the pressure in the tank to 5 bar.
- Relevant Equations
- dU=dQ-dW-dmh
Hi there. I have a problem solving above problem. How can I move on with my solution? It seems to me that I have proper approach but I'm just stuck with the energy equation ;/
Part of solution below:
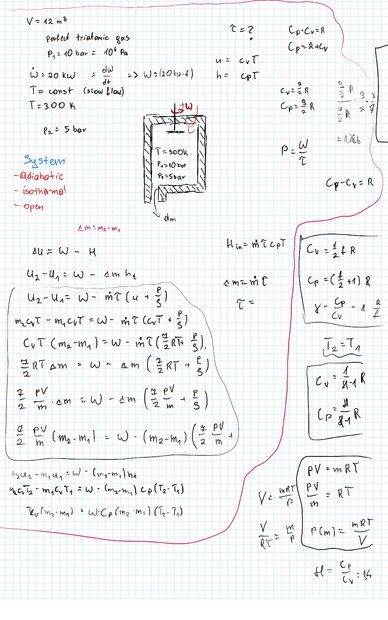
Part of solution below: