- #1
faiziqb12
Gold Member
- 105
- 3
let's look at force at the atomic level to understand the Newtons third law of motion. I'll use Helium atoms as an example.
Now imagine we start with one atom HE2 stationary, and throw another atom HE1 at it.It is the velocity of HE1 that affects the motion of HE2 , because the system of these two HE2 atoms is isolated , and there is no unbalanced force on each of these . According to physics both the atoms will experience action reaction forces at the same time .
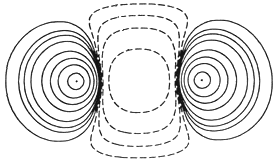
for sake of simplicity let's consider a imaginary hollow box between the two atoms as an overlapping of their atomic forces. As soon as HE1 moves x distance inside this box , there will be a increase of y amount of velocity in HE2 and a correspoding decrease of y in the velocity of HE1 . But there is still a greater velocity in HE1 as compared to HE2 , so the process of changing of velocities will continue up to the time when both the atoms have the same amount of velocity , because after they attain the same amount of velocity they won't be able to enter tat box again .
So , is what i just explained right ?
i don't think so, because we know from our knowledge of head on elastic collisions of equal masses that when HE1 and HE2 will collide, HE2 will gain the velocity that HE1 had and HE1 will itself become stationary.
so can you please correct me , and provide me with the correct answer to understand the Newtons third law of motion ?
Now imagine we start with one atom HE2 stationary, and throw another atom HE1 at it.It is the velocity of HE1 that affects the motion of HE2 , because the system of these two HE2 atoms is isolated , and there is no unbalanced force on each of these . According to physics both the atoms will experience action reaction forces at the same time .
for sake of simplicity let's consider a imaginary hollow box between the two atoms as an overlapping of their atomic forces. As soon as HE1 moves x distance inside this box , there will be a increase of y amount of velocity in HE2 and a correspoding decrease of y in the velocity of HE1 . But there is still a greater velocity in HE1 as compared to HE2 , so the process of changing of velocities will continue up to the time when both the atoms have the same amount of velocity , because after they attain the same amount of velocity they won't be able to enter tat box again .
So , is what i just explained right ?
i don't think so, because we know from our knowledge of head on elastic collisions of equal masses that when HE1 and HE2 will collide, HE2 will gain the velocity that HE1 had and HE1 will itself become stationary.
so can you please correct me , and provide me with the correct answer to understand the Newtons third law of motion ?