John37309
- 102
- 0
Are Cyclobutane chains possible? that's my question.
At least that's what i have been calling these chains.
This is 1 normal Cyclobutane; C4H8
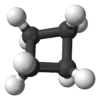
This might be 2 Cyclobutane's chained together; C7H12

This might be 3 Cyclobutane's chained together; C10H16

------
So I'm asking the question is it possible to form these chains? I can't find any information about these molecules. But maybe its because I'm calling them the wrong thing. Maybe these hydrocarbons have a special name. Or does anyone know why i can't seem to find any info about these type of molecules? I have suggested a chain of 2 Cyclobutane's or 3 Cyclobutane's, but in theory, maybe its possible for many more of these molecules to chain together?, Maybe 6 or 7 of them chained together in a similar fashion.
Thanks Guys,
John.
At least that's what i have been calling these chains.
This is 1 normal Cyclobutane; C4H8
This might be 2 Cyclobutane's chained together; C7H12
This might be 3 Cyclobutane's chained together; C10H16
------
So I'm asking the question is it possible to form these chains? I can't find any information about these molecules. But maybe its because I'm calling them the wrong thing. Maybe these hydrocarbons have a special name. Or does anyone know why i can't seem to find any info about these type of molecules? I have suggested a chain of 2 Cyclobutane's or 3 Cyclobutane's, but in theory, maybe its possible for many more of these molecules to chain together?, Maybe 6 or 7 of them chained together in a similar fashion.
Thanks Guys,
John.