1msm
- 6
- 0
Hello everyone !
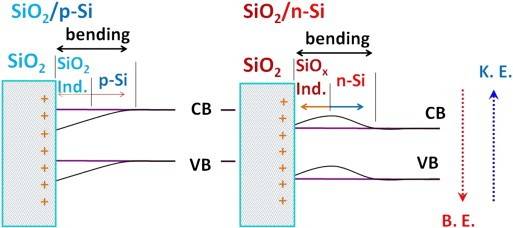
In below picture we can see band bending in semiconductor at SiO2 interface,
as show in picture how charge's BE(binding energy) and KE(kinetic energy) are changing..??
Or what happens to charges total energy in band bending ..??
\\
In below picture we can see band bending in semiconductor at SiO2 interface,
as show in picture how charge's BE(binding energy) and KE(kinetic energy) are changing..??
Or what happens to charges total energy in band bending ..??
\\