- #1
Moogie
- 168
- 1
Hi
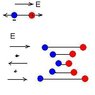
I'm a little bit confused about infrared spectroscopy. My basic understanding (which was obviously wrong) was that molecules absorbed EMR in the infrared region and that this caused vibrations in the molecule. Some vibrations/stretching cause a change in charge distribution and for reasons not to known to me, only those that cause a change in charge show up on the IR spectra.
However I have just read:
Not all molecular vibrations lead to observable infrared absorptions. In general, a vibration must cause a change in the charge distribution within a molecule to absorb infrared light. The greater the change in charge distribution, the stronger the absorption.
This explains why the spectra only shows vibrations that cause a change in charge, but what I don't understand is why "not all vibrations lead to observable infrared absorbtions." How can any vibration occur without absorbing energy. I thought the molecules absorbed the energy which caused the vibration, not that the vibration caused energy to be absorbed.
I would appreciate it if someone could clarify this for me
Kind regards
I'm a little bit confused about infrared spectroscopy. My basic understanding (which was obviously wrong) was that molecules absorbed EMR in the infrared region and that this caused vibrations in the molecule. Some vibrations/stretching cause a change in charge distribution and for reasons not to known to me, only those that cause a change in charge show up on the IR spectra.
However I have just read:
Not all molecular vibrations lead to observable infrared absorptions. In general, a vibration must cause a change in the charge distribution within a molecule to absorb infrared light. The greater the change in charge distribution, the stronger the absorption.
This explains why the spectra only shows vibrations that cause a change in charge, but what I don't understand is why "not all vibrations lead to observable infrared absorbtions." How can any vibration occur without absorbing energy. I thought the molecules absorbed the energy which caused the vibration, not that the vibration caused energy to be absorbed.
I would appreciate it if someone could clarify this for me
Kind regards