Discussion Overview
The discussion centers around the implications of Bohmian Mechanics in the context of the double slit experiment, particularly regarding the paths of photons and whether they can be said to travel faster than the speed of light. Participants explore theoretical interpretations, the compatibility of Bohmian Mechanics with special relativity, and the nature of photon trajectories.
Discussion Character
- Exploratory
- Debate/contested
- Technical explanation
Main Points Raised
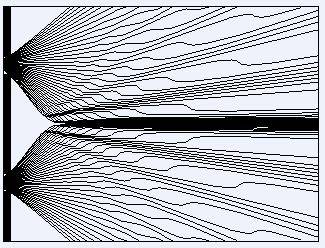
- Some participants propose that in Bohmian Mechanics, photons may take non-straight paths from the slits to the detector, suggesting that if the time taken for a photon to travel is measured, it could imply either a straight-line trajectory or a speed greater than c.
- Others argue that Bohmian Mechanics is not applicable to photons, as it is fundamentally incompatible with special relativity and does not account for developments in Quantum Field Theory (QFT).
- A participant cites a paper claiming that Bohmian Mechanics can be observationally compatible with relativity, despite challenges in achieving Lorentz invariance.
- Some contributions highlight that time-of-flight questions are not meaningful in standard quantum mechanics, as particles do not have defined trajectories until measured, a concept that is more applicable within Bohmian Mechanics.
- There are claims that Bohmian velocities of photons may exceed c in certain contexts, but this does not pose a problem as measurements yield velocities equal to c.
- Several participants reference academic papers to support their views on the compatibility of Bohmian Mechanics with relativistic principles and the nature of photon trajectories.
Areas of Agreement / Disagreement
Participants express multiple competing views regarding the applicability of Bohmian Mechanics to photons and its compatibility with special relativity. The discussion remains unresolved, with no consensus on the interpretations of photon trajectories or the implications of Bohmian Mechanics.
Contextual Notes
Some participants note that Bohmian Mechanics may not adequately describe photons due to its non-Lorentz invariant nature, while others suggest that it can be modified or that its implications are still relevant in certain contexts. The discussion reflects a range of assumptions and interpretations regarding the nature of quantum mechanics and its foundational principles.