Jay Macarus
- 7
- 0
Hey guys, can't seem to make sense of this question from phys. It goes..
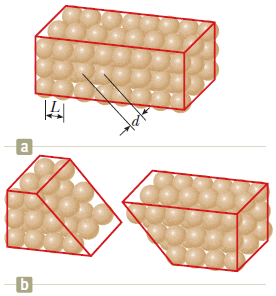
"A crystalline solid consists of atoms stacked up in a repeating lattice structure. Consider a crystal as shown in Figure a. The atoms reside at the corners of cubes of side L = 0.200 nm.
One piece of evidence for the regular arrangement of atoms comes from the flat surfaces along which a crystal separates, or cleaves, when it is broken. Suppose this crystal cleaves along a face diagonal, as shown in Figure b. Calculate the spacing d between two adjacent atomic planes that separate when the crystal cleaves."
I considered using the visual representation from the figure, but I'm sure I'm just overlooking a simple solution. I'm not honestly sure what its looking for me to do here.
Thanks for the help!
-Jay
"A crystalline solid consists of atoms stacked up in a repeating lattice structure. Consider a crystal as shown in Figure a. The atoms reside at the corners of cubes of side L = 0.200 nm.
One piece of evidence for the regular arrangement of atoms comes from the flat surfaces along which a crystal separates, or cleaves, when it is broken. Suppose this crystal cleaves along a face diagonal, as shown in Figure b. Calculate the spacing d between two adjacent atomic planes that separate when the crystal cleaves."
I considered using the visual representation from the figure, but I'm sure I'm just overlooking a simple solution. I'm not honestly sure what its looking for me to do here.
Thanks for the help!
-Jay