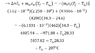Homework Help Overview
The discussion revolves around calculating the initial temperature of an iron block that has been submerged in water. Participants are examining the thermal interactions and specific heat capacities involved in the problem.
Discussion Character
Approaches and Questions Raised
- Participants are sharing their calculated initial temperatures and questioning the accuracy of their results based on differing inputs and assumptions. There is a focus on the significance of rounding and the precision of measurements.
Discussion Status
The conversation is ongoing, with participants actively comparing their calculations and seeking clarification on specific values and conventions. Some have offered insights into potential errors and the importance of consistent significant figures, while others have acknowledged discrepancies in their results.
Contextual Notes
There are mentions of varying specific heat capacities and the need for precise measurements, as well as concerns regarding the sign convention used in calculations. Participants are also discussing the implications of measurement precision on their results.