dpage
- 6
- 0
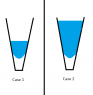
Suppose we have a very small V shaped tube-like vessel that is closed at the bottom (see attached picture).
Question 1:
If a drop of liquid enters the vessel from the top, what determines the stopping point of the drop (i.e. at what point is the force of gravity countered and the drop stops...?). I imagine this equation would include the radius of the tube at any given vertical value, along with the contact angle of the liquid with the solid...Is there such an equation?
Question 2:
Suppose you have added a drop as described in question 1, but after the drop stops moving down you add more liquid on top. Will the drop sink further down the hole as a function of how much liquid is in the vessel?
The attached figure depicts questions 1 and 2 as Case 1 and Case 2, respectively.
thanks
Question 1:
If a drop of liquid enters the vessel from the top, what determines the stopping point of the drop (i.e. at what point is the force of gravity countered and the drop stops...?). I imagine this equation would include the radius of the tube at any given vertical value, along with the contact angle of the liquid with the solid...Is there such an equation?
Question 2:
Suppose you have added a drop as described in question 1, but after the drop stops moving down you add more liquid on top. Will the drop sink further down the hole as a function of how much liquid is in the vessel?
The attached figure depicts questions 1 and 2 as Case 1 and Case 2, respectively.
thanks