dtwright03
- 2
- 1
I work in R&D for coatings and have a bit of a plumbing problem on my pilot coater. I've formulated a very reactive mix that, once mixed, increases in viscosity by 3000cP an hour. This makes it very difficult to get a constant roll of sample material to send to customers with the equipment that I have available.
SO, I want to get a 1/2"x11" static mixer and use two feed streams to resolve this issue. My question should be simple to a trained professional. If I have one air line feeding a pressure pot 10psi and another feeding a second pressure pot 20psi, would you expect that converging these lines into a static mixer would create significant backflow at a final flow rate around 0.01gpm venting to atmosphere? Both mixes differ in viscosity by 5000cP. Alternatively, is there a valve that prevents backflow of both air and liquid to give me peace of mind that the proportions are being fed correctly?
I sadly don't have $1000 for a couple of flowmeters...Here's a handy dandy picture.
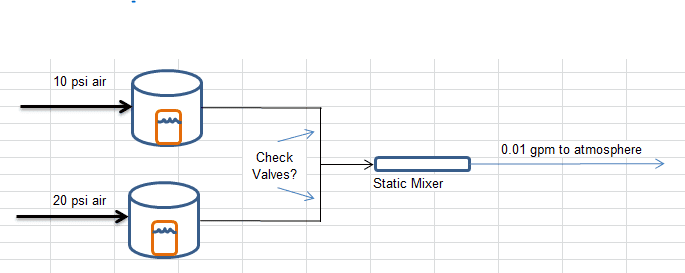
SO, I want to get a 1/2"x11" static mixer and use two feed streams to resolve this issue. My question should be simple to a trained professional. If I have one air line feeding a pressure pot 10psi and another feeding a second pressure pot 20psi, would you expect that converging these lines into a static mixer would create significant backflow at a final flow rate around 0.01gpm venting to atmosphere? Both mixes differ in viscosity by 5000cP. Alternatively, is there a valve that prevents backflow of both air and liquid to give me peace of mind that the proportions are being fed correctly?
I sadly don't have $1000 for a couple of flowmeters...Here's a handy dandy picture.