- 19,772
- 10,723
Chemists claim to have solved riddle of how life began on Earth
http://phys.org/news/2015-03-chemists-riddle-life-began-earth.html
Here is the journal article
http://www.nature.com/nchem/journal/vaop/ncurrent/full/nchem.2202.html
http://phys.org/news/2015-03-chemists-riddle-life-began-earth.html
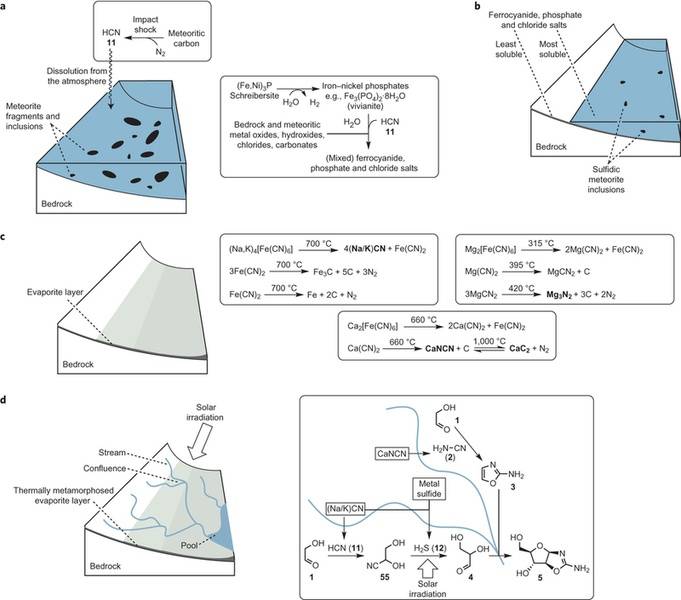
A team of chemists working at the MRC Laboratory of Molecular Biology, at Cambridge in the UK believes they have solved the mystery of how it was possible for life to begin on Earth over four billion years ago. In their paper published in the journal Nature Chemistry, the team describes how they were able to map reactions that produced two and three-carbon sugars, amino acids, ribonucleotides and glycerol—the material necessary for metabolism and for creating the building blocks of proteins and ribonucleic acid molecules and also for allowing for the creation of lipids that form cell membranes.
Here is the journal article
http://www.nature.com/nchem/journal/vaop/ncurrent/full/nchem.2202.html
Last edited: