Assim
- 3
- 1
I have a project which i need to cool down certain quantity of hot water.
I need to cool down water of around 25 litres at 50 Degree celcius to 30 Degree celcius, Static water, not flowing. (or incoming 50 Degrees - Outgoing 30 Degrees with approx 1.5 gallons per minute flow rate)
time available to cool down the water -10 to 15 mins
I imagined I will be able to cool down the water with a regular water chiller unit with compressor and immersed coil arrangement (pics below). I conducted that experiment but it failed. The cooling doesn't works as expected
I am an armature with water cooling and such physics.
Cold anybody suggest me some method for the above problem.
Thanks
my experiment arrangement
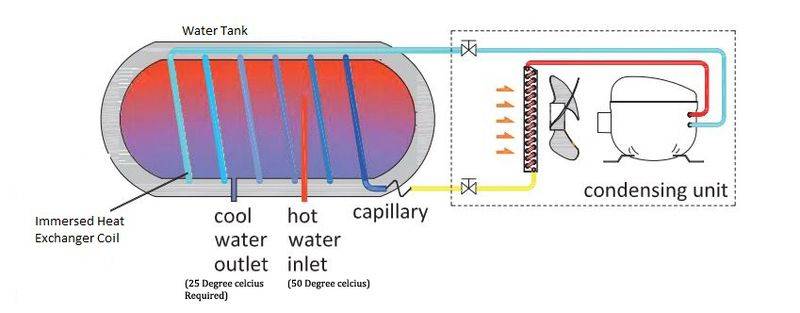
I need to cool down water of around 25 litres at 50 Degree celcius to 30 Degree celcius, Static water, not flowing. (or incoming 50 Degrees - Outgoing 30 Degrees with approx 1.5 gallons per minute flow rate)
time available to cool down the water -10 to 15 mins
I imagined I will be able to cool down the water with a regular water chiller unit with compressor and immersed coil arrangement (pics below). I conducted that experiment but it failed. The cooling doesn't works as expected
I am an armature with water cooling and such physics.
Cold anybody suggest me some method for the above problem.
Thanks
my experiment arrangement
Last edited by a moderator: