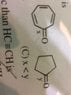
Discussion Overview
The discussion revolves around determining the correct order of bond lengths in two organic molecules, with a focus on the influence of resonance on bond stability and length. Participants explore concepts related to bond length, resonance, and the application of Hückel's rules.
Discussion Character
- Homework-related, Conceptual clarification, Debate/contested
Main Points Raised
- One participant suggests that molecule x participates in resonance, leading to the conclusion that x is more stable than y, thus proposing that x
- Another participant inquires about the application of Hückel's rules, indicating a potential relevance to the bond length discussion.
- Responses indicate familiarity with the 4n + 2 π electron rule, but there is uncertainty about its application in this specific context.
- One participant asserts that more resonance correlates with longer bond lengths, but this claim is met with a request for clarification on what it is being compared to.
Areas of Agreement / Disagreement
Participants express differing views on the relationship between resonance and bond length, with some asserting that more resonance leads to longer bonds while others seek clarification on this point. The discussion remains unresolved regarding the correct order of bond lengths.
Contextual Notes
There are unresolved assumptions regarding the definitions of stability and resonance in the context of bond lengths, as well as the specific application of Hückel's rules to the molecules in question.