christian everett
- 16
- 2
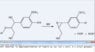
I am guessing that just pure hydrogen sulfide, gaseous or liquified under pressure without the sodium, would effect the depolymerization of lignin which is the goal of the Kraft process.
Hydrogen sulfide can be produced by treating hydrogen directly with molten elemental sulfur at about 450 °C, which I am guessing would be a lower temperature for less time, saving energy than the production of sodium sulfide from sodium sulfate and carbon.
Also there would be less materials, saving shipping costs (since one mole of hydrogen sulfide weighs 34 grams and one mole of sodium sulfate weighs 142 grams plus two moles of carbon weighing 24 grams totaling 166 grams.
Hydrogen sulfide can be produced by treating hydrogen directly with molten elemental sulfur at about 450 °C, which I am guessing would be a lower temperature for less time, saving energy than the production of sodium sulfide from sodium sulfate and carbon.
Also there would be less materials, saving shipping costs (since one mole of hydrogen sulfide weighs 34 grams and one mole of sodium sulfate weighs 142 grams plus two moles of carbon weighing 24 grams totaling 166 grams.