aname
- 8
- 0
- Homework Statement
- i'm not sure I used the numbers right
- Relevant Equations
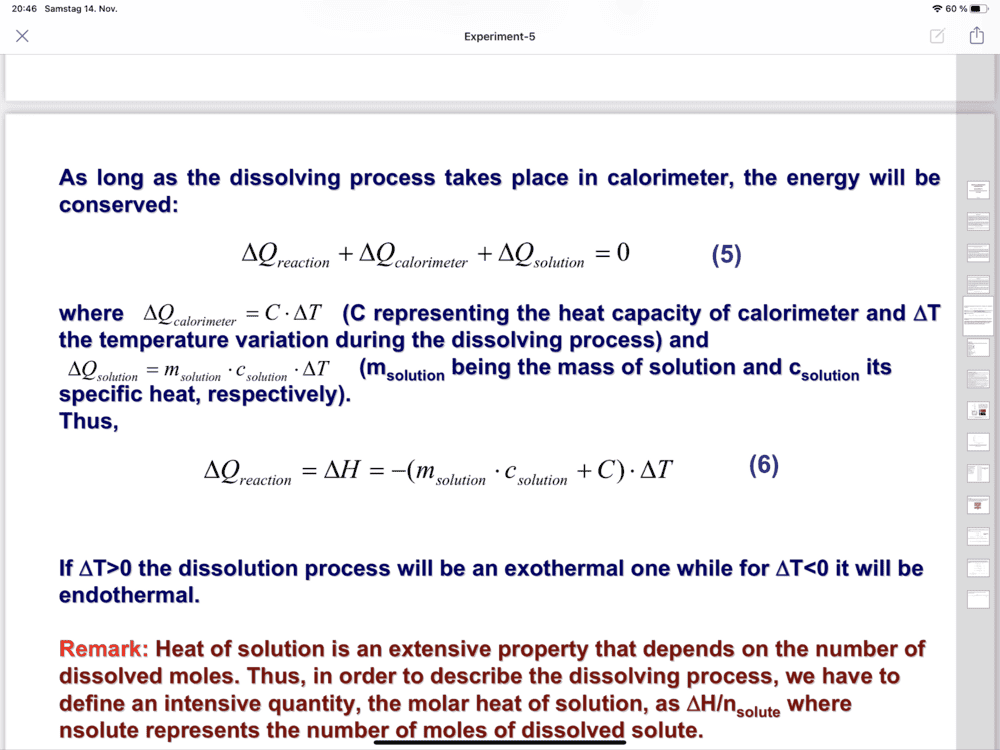
- Determination of the dissolution enthalpy
-(7.455 so,94+8,4) Delta T
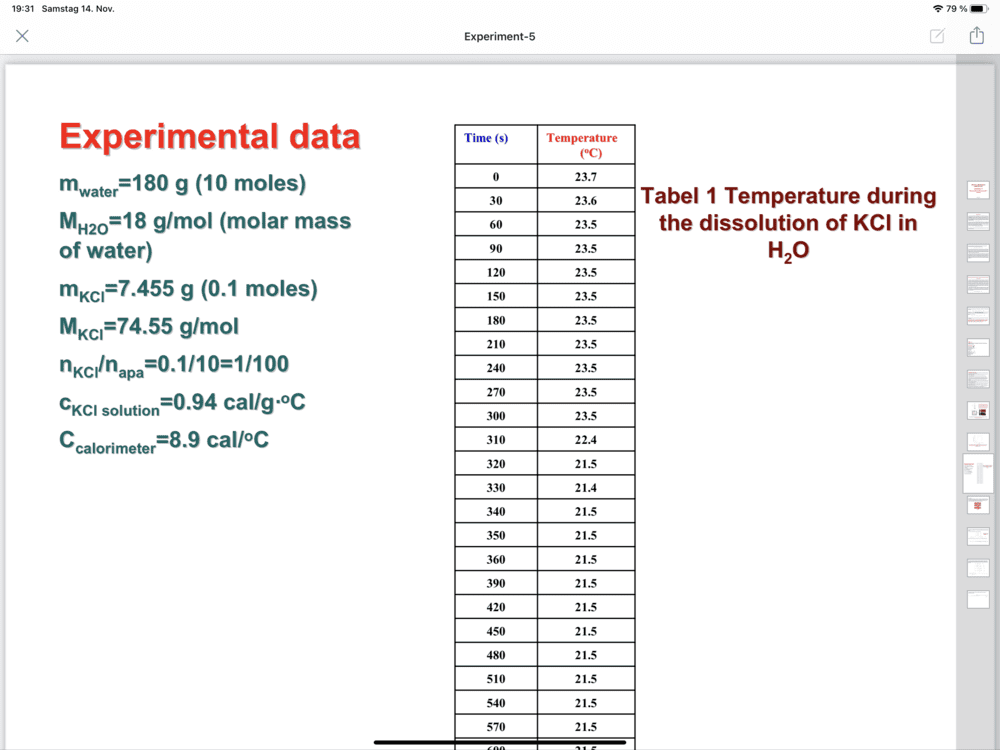
Hi,Chestermiller said:No. m of the solution is 187.5 g.