rwooduk
- 757
- 59
This relates to a question I asked recently on Quantum Dots, but I'll rephrase it and hopefully any chemists out there can help.
If we have (n,l) = (1,2) where n and l are quantum numbers can we determine the orbitals? and hence the number of electrons in a quantum dot?
i.e.
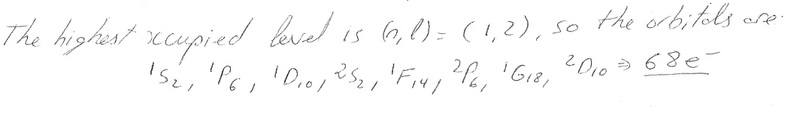
And also I've always used the SSPSP... to fill orbitals, what's happened to the second S etc? why does he go SPDSF
Thanks in advance for any help, completely lost.
If we have (n,l) = (1,2) where n and l are quantum numbers can we determine the orbitals? and hence the number of electrons in a quantum dot?
i.e.
And also I've always used the SSPSP... to fill orbitals, what's happened to the second S etc? why does he go SPDSF
Thanks in advance for any help, completely lost.