- #1
tade
- 702
- 24
For the Blackbody Spectrum, there are two versions of the formula, one for wavelength and the other one for frequency:
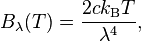
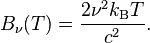
The peak intensities for both occur at different wavelengths (or frequencies).How do scientists measure the spectral radiance of blackbodies?
Are there TWO types of equipment, one for [tex]B_\lambda[/tex] and the other for [tex]B_\nu[/tex], such that each device yields a peak at a different frequency?
The peak intensities for both occur at different wavelengths (or frequencies).How do scientists measure the spectral radiance of blackbodies?
Are there TWO types of equipment, one for [tex]B_\lambda[/tex] and the other for [tex]B_\nu[/tex], such that each device yields a peak at a different frequency?