Sunwoo Bae
- 60
- 4
- Homework Statement
- Draw the important resonance forms for SO42-
- Relevant Equations
- none
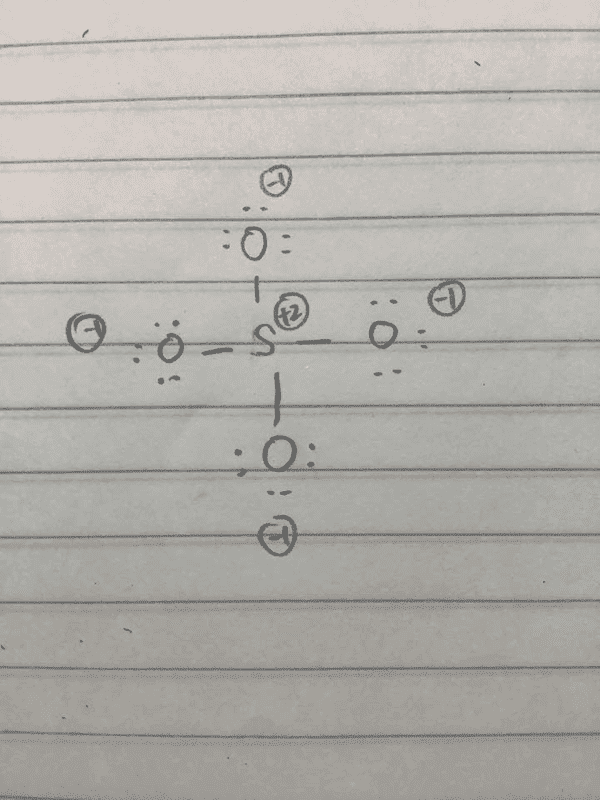
I was told that there are six resonance structures for SO42-, as shown below.

I am wondering why this structure with single bonds is not one of the possible resonance.
I understand that it is unfavorable as the formal charges are spread out over all four atoms, but shouldn't is still be a part of the resonance, given that the total formal charge of the molecule adds up to 2- in total?
Thank you
I am wondering why this structure with single bonds is not one of the possible resonance.
I understand that it is unfavorable as the formal charges are spread out over all four atoms, but shouldn't is still be a part of the resonance, given that the total formal charge of the molecule adds up to 2- in total?
Thank you
Last edited by a moderator: