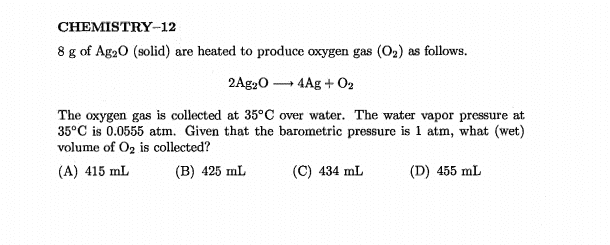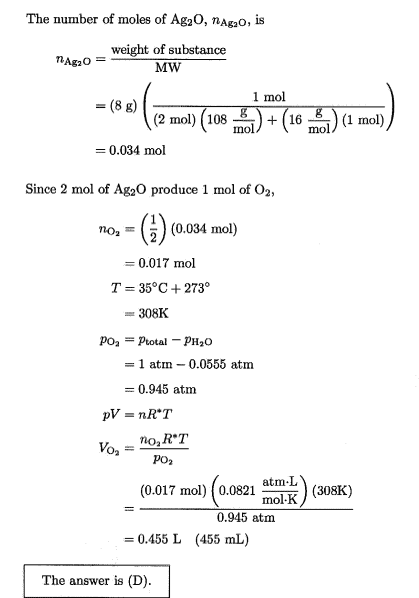SUMMARY
The discussion centers on calculating dry and wet volumes of gas mixtures using the Ideal Gas Law, specifically in a scenario involving a container of water vapor and oxygen. The correct dry volume is determined to be 436 mL, while the wet volume is 462 mL, based on the pressure contributions of water vapor and oxygen. Participants clarify that when calculating dry volume, the pressure due to water vapor (0.0555 atm) is not subtracted from the total pressure, which is 1 atm. Miscalculations arise from rounding errors in intermediate steps, leading to discrepancies in results.
PREREQUISITES
- Understanding of the Ideal Gas Law and its application
- Familiarity with Dalton's Law of Partial Pressures
- Knowledge of significant figures in scientific calculations
- Basic concepts of gas mixtures and vapor pressure
NEXT STEPS
- Study the Ideal Gas Law calculations for gas mixtures
- Learn about Dalton's Law and its implications in gas pressure calculations
- Explore the concept of significant figures and their importance in scientific measurements
- Investigate the effects of water vapor on gas volume calculations
USEFUL FOR
Chemistry students, laboratory technicians, and anyone involved in gas volume calculations or studying gas laws in physical chemistry.