1msm
- 6
- 0
Hello everyone,
here we have two types of electron tunneling paths of electrons.
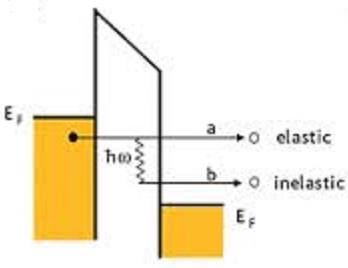
as shown here, In an elastic tunneling the electron goes directly into metal2 with out
falling to Fermi level of metal2..?? or Is it going to fallback by radiating its energy..??
here we have two types of electron tunneling paths of electrons.
as shown here, In an elastic tunneling the electron goes directly into metal2 with out
falling to Fermi level of metal2..?? or Is it going to fallback by radiating its energy..??