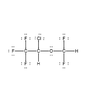
Enflurane (CHFClCF2OCHF2) Lewis structure
- Thread starter andkand97
- Start date
Click For Summary
SUMMARY
The discussion centers on the Lewis structure of Enflurane (CHFClCF2OCHF2). Participants clarify the distinction between "tri-fluoro-methyl" and "di-fluoro-methyl" groups, which is crucial for accurately representing the molecular structure. The correct identification of these groups directly impacts the understanding of Enflurane's chemical properties and reactivity. Accurate Lewis structures are essential for predicting molecular behavior in chemical reactions.
PREREQUISITES- Understanding of Lewis structures and molecular geometry
- Knowledge of functional groups, specifically halogenated hydrocarbons
- Familiarity with chemical nomenclature, particularly regarding fluorinated compounds
- Basic principles of organic chemistry
- Study the characteristics of halogenated hydrocarbons
- Learn about the implications of substituent groups in organic chemistry
- Research the properties and applications of Enflurane in medicinal chemistry
- Explore advanced topics in molecular modeling and visualization tools
Chemistry students, organic chemists, and professionals involved in pharmaceutical research will benefit from this discussion, particularly those focusing on molecular structure and reactivity of fluorinated compounds.
Similar threads
- · Replies 19 ·
- · Replies 5 ·
- · Replies 4 ·
- · Replies 1 ·
- · Replies 1 ·
- · Replies 9 ·
- · Replies 7 ·