- #1
Angella
- 2
- 0
We Now From Information Theory That Entropy Of Functions Of A Random Variable X Is Less Than Or Equal To The Entropy Of X.
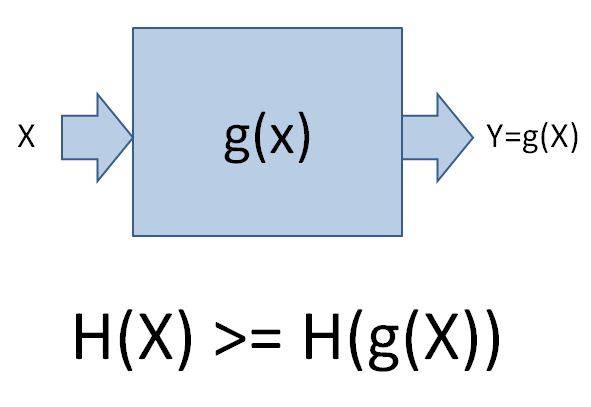
Does It Break The Second Law Of Thermodynamic?
Does It Break The Second Law Of Thermodynamic?