unscientific
- 1,728
- 13
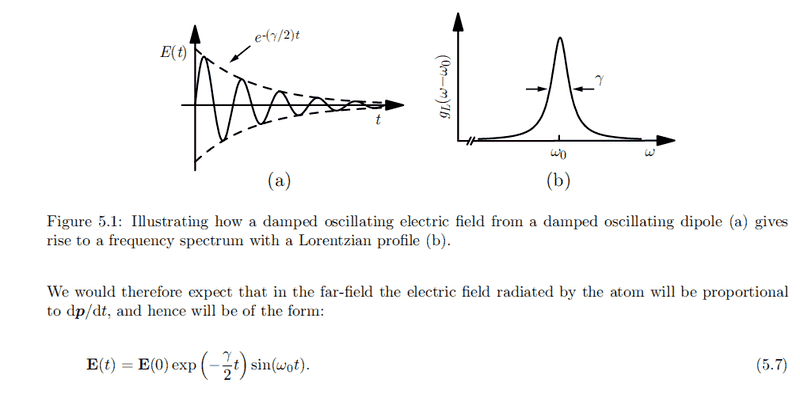
For an EM wave close to the transition frequency ##\omega_{21}##, we assume the dipole moment to be exponentially damped and oscillating:
p(t) = p(0) e^{-\frac{\gamma}{2}t} cos(\omega_0 t)
Why do we expect the electric field to be proportional to ##\dot p##?
Taken from my lecturer notes on laser and atomic physics:
p(t) = p(0) e^{-\frac{\gamma}{2}t} cos(\omega_0 t)
Why do we expect the electric field to be proportional to ##\dot p##?
Taken from my lecturer notes on laser and atomic physics: