pyroknife
- 611
- 4
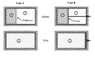
I attached an image of two expansion cases I am analyzing. Both cases involve an insulated cylinder that is divided by a separating element (diaphragm for case A and piston for B). The portion on the right is evacuated. The left contains a calorically perfect gas with an initial pressure and temperature.
Case A is a thin diaphragm and at a certain point in time, it is suddenly removed (removed without friction&flow disturbance) and gas expands to fill the whole cylinder. Case B is a piston which is allowed to move (without friction) while gas expands to fill the whole cylinder.
Would both of these cases be considered free expansion?
Case A is a thin diaphragm and at a certain point in time, it is suddenly removed (removed without friction&flow disturbance) and gas expands to fill the whole cylinder. Case B is a piston which is allowed to move (without friction) while gas expands to fill the whole cylinder.
Would both of these cases be considered free expansion?