- #1
Heart
- 39
- 0
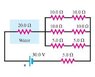
"In the circuit in the figure, a 20-ohm resistor sits inside 102 g of pure water that is surrounded by insulating Styrofoam."
"If the water is initially at temperature 10.1 deg. celsius, how long will it take for its temperature to rise to 58.9 deg. celsius?
Use as the heat capacity of water, and express your answer in seconds using three significant figures."
What I did was finding the total resistance to be equal to 30 ohms.
I then use the mc(delta t) formula; the energy needed is then equal to 20856.144 J. I then equated this to P=V^2/R. I then found the time to be ~ 656 seconds. I'm pretty sure I did it right but MP said I did it wrong. Any suggestions?
Thanks in advance,
"If the water is initially at temperature 10.1 deg. celsius, how long will it take for its temperature to rise to 58.9 deg. celsius?
Use as the heat capacity of water, and express your answer in seconds using three significant figures."
What I did was finding the total resistance to be equal to 30 ohms.
I then use the mc(delta t) formula; the energy needed is then equal to 20856.144 J. I then equated this to P=V^2/R. I then found the time to be ~ 656 seconds. I'm pretty sure I did it right but MP said I did it wrong. Any suggestions?
Thanks in advance,