Feynmanfan
- 128
- 0
Dear friends,
I’m having trouble with this thermodynamic problem. I apologise if you don’t understand my poor English (I’m writing to you from Spain!)
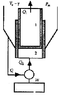
We’ve got two cylinders (1 is adiabatically isolated from 2 but not from the outside, where Pressure P and Tº are constant) and cylinder 1 can move inside cylinder 2. Cylinder 2 is isolated from the outside but it is connected to a heat pump
Suppose both cylinders contain IDEAL GAS.
Everything is in equilibrium but now we lower Tº and make it (T-C).Therefore Q1 heat will be delivered from cylinder 1. As said, Cylinder 2 is connected to a Carnot HEAT PUMP (that is maximum efficiency) and we use Q1 to heat up cylinder 2.
I need to calculate the volume of the gas in cylinder 2, entropy change in both systems and many other things I don’t want to bore you with.
Does this problem have to do with thermodynamic potentials? I’d be very grateful if you could help me.
I’m having trouble with this thermodynamic problem. I apologise if you don’t understand my poor English (I’m writing to you from Spain!)
We’ve got two cylinders (1 is adiabatically isolated from 2 but not from the outside, where Pressure P and Tº are constant) and cylinder 1 can move inside cylinder 2. Cylinder 2 is isolated from the outside but it is connected to a heat pump
Suppose both cylinders contain IDEAL GAS.
Everything is in equilibrium but now we lower Tº and make it (T-C).Therefore Q1 heat will be delivered from cylinder 1. As said, Cylinder 2 is connected to a Carnot HEAT PUMP (that is maximum efficiency) and we use Q1 to heat up cylinder 2.
I need to calculate the volume of the gas in cylinder 2, entropy change in both systems and many other things I don’t want to bore you with.
Does this problem have to do with thermodynamic potentials? I’d be very grateful if you could help me.