rice421
- 2
- 0
Hi Everyone, Hope this is the right section of the forum.
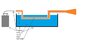
I need some help to design a small tube heater for a specific application. I have attached a crude picture to show what i need. The flame needs to travel through a 3/4 inch pipe about 7 feet to heat the water. The problem is that i don't want to use any power so i can't use a blower or fan. I hope to use the venturi effect or some other way to get the flame to suck in enough air to burn hot and reliably with no power. Not sure how I am going to ignite it yet but that should be the easy part.
Any ideas on how to get this flame to burn in a closed space with no fan??
Thanks in advance for your help
I need some help to design a small tube heater for a specific application. I have attached a crude picture to show what i need. The flame needs to travel through a 3/4 inch pipe about 7 feet to heat the water. The problem is that i don't want to use any power so i can't use a blower or fan. I hope to use the venturi effect or some other way to get the flame to suck in enough air to burn hot and reliably with no power. Not sure how I am going to ignite it yet but that should be the easy part.
Any ideas on how to get this flame to burn in a closed space with no fan??
Thanks in advance for your help