asdfTT123
- 9
- 0
I've been staring at these for a while now and I can't seem to get them. I would really appreciate anyone's help with these.
1. Methylcyclohexane -> 1-methyl,2-bromo-cyclohexane (bromine added to ortho carbon in respect to the methyl group). The main problem here is that I don't know where to start!
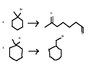
2, 3. The rest are on the attachment.
Thanks!
1. Methylcyclohexane -> 1-methyl,2-bromo-cyclohexane (bromine added to ortho carbon in respect to the methyl group). The main problem here is that I don't know where to start!
2, 3. The rest are on the attachment.
Thanks!