Marconis
- 5
- 0
Draw Newman projections depicting the (R)-enantiomer of each of the following:
a) 1-bromo-1-chloroethane
b) 1-bromo-1-methoxypropane
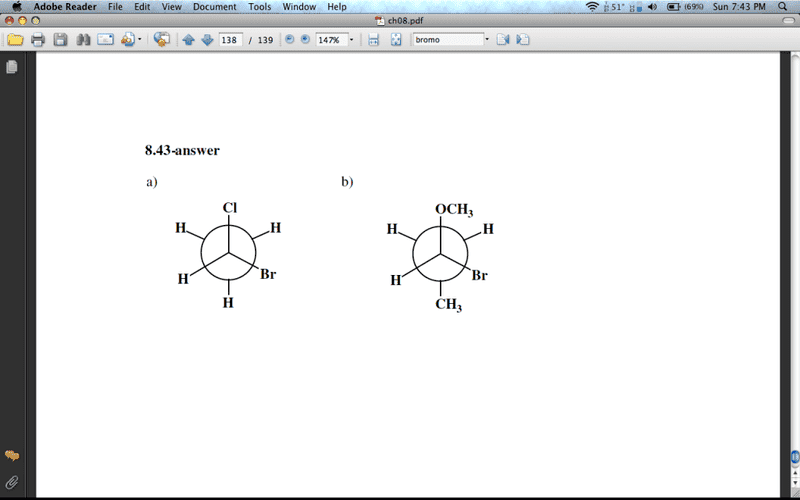
Now, I am SURE that I am mistaken, but isn't that an (S)-enantiomer for each? Assigning priorities:
For a) Br > Cl > CH3 > H...right? So, wouldn't the (R)-enantiomer have the Br and Cl switched? With Br on the top, you have priority 1 at the top, 2 at the bottom right, 3 in the middle, and 4 on the bottom left. Isn't that a clockwise direction, with 4th priority facing away from us? Or, is that H facing towards us...help!
Same goes for b, I switched the OCH3 and the Br...Something is really messing me up that is causing me to do this. Thank you in advance!
a) 1-bromo-1-chloroethane
b) 1-bromo-1-methoxypropane
Now, I am SURE that I am mistaken, but isn't that an (S)-enantiomer for each? Assigning priorities:
For a) Br > Cl > CH3 > H...right? So, wouldn't the (R)-enantiomer have the Br and Cl switched? With Br on the top, you have priority 1 at the top, 2 at the bottom right, 3 in the middle, and 4 on the bottom left. Isn't that a clockwise direction, with 4th priority facing away from us? Or, is that H facing towards us...help!
Same goes for b, I switched the OCH3 and the Br...Something is really messing me up that is causing me to do this. Thank you in advance!